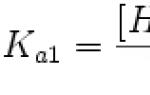Cheat sheet hormone enzymes. Fundamentals of Lehninger's biochemistry Methods for regulating metabolic activity in a cell
DYNAMIC BIOCHEMISTRY
ChapterIV.8.
Metabolism and energy
Metabolism or metabolism - a set of chemical reactions in the body that provide it with the substances and energy necessary for life. In metabolism, two main stages can be distinguished: preparatory - when a substance received through the alimentary route undergoes chemical transformations, as a result of which it can enter the blood and then penetrate into cells, and metabolism itself, i.e. chemical transformations of compounds that have penetrated into cells.
Metabolic pathway - this is the nature and sequence of chemical transformations of a specific substance in the body. Intermediate products formed during the metabolic process are called metabolites, and the last compound of the metabolic pathway is the final product.
The process of breaking down complex substances into simpler ones is called catabolism. Thus, proteins, fats, and carbohydrates in food are broken down into simpler components (amino acids, fatty acids and monosaccharides) under the action of enzymes in the digestive tract. This releases energy. The reverse process, i.e. the synthesis of complex compounds from simpler ones is called anabolism . It comes with an expenditure of energy. From amino acids, fatty acids and monosaccharides formed as a result of digestion, new cellular proteins, membrane phospholipids and polysaccharides are synthesized in cells.
There is a concept amphibolism when one compound is destroyed, but another is synthesized.
Metabolic cycle is a metabolic pathway in which one of the end products is identical to one of the compounds involved in this process.
A particular metabolic pathway is a set of transformations of one specific compound (carbohydrates or proteins). The general metabolic pathway is when two or more types of compounds are involved (carbohydrates, lipids and partially proteins are involved in energy metabolism).
Metabolic substrates - compounds supplied with food. Among them, there are main nutrients (proteins, carbohydrates, lipids) and minor ones, which come in small quantities (vitamins, minerals).
The intensity of metabolism is determined by the cell’s need for certain substances or energy; regulation is carried out in four ways:
1) The total reaction rate of a particular metabolic pathway is determined by the concentration of each of the enzymes in this pathway, the pH value of the environment, the intracellular concentration of each of the intermediate products, and the concentration of cofactors and coenzymes.
2) The activity of regulatory (allosteric) enzymes, which usually catalyze the initial stages of metabolic pathways. Most of them are inhibited by the end product of this pathway and this type of inhibition is called "feedback".
3) Genetic control that determines the rate of synthesis of a particular enzyme. A striking example is the appearance of inducible enzymes in a cell in response to the supply of a corresponding substrate.
4) Hormonal regulation. A number of hormones can activate or inhibit many enzymes in metabolic pathways.
Living organisms are thermodynamically unstable systems. For their formation and functioning, a continuous supply of energy is required in a form suitable for multifaceted use. To obtain energy, almost all living beings on the planet have adapted to hydrolyze one of the pyrophosphate bonds of ATP. In this regard, one of the main tasks of the bioenergetics of living organisms is the replenishment of used ATP from ADP and AMP.
The main source of energy in the cell is the oxidation of substrates with atmospheric oxygen. This process occurs in three ways: the addition of oxygen to the carbon atom, the abstraction of hydrogen, or the loss of an electron. In cells, oxidation occurs in the form of a sequential transfer of hydrogen and electrons from the substrate to oxygen. In this case, oxygen plays the role of a reducing compound (oxidizing agent). Oxidative reactions occur with the release of energy. Biological reactions are characterized by relatively small changes in energy. This is achieved by splitting the oxidation process into a number of intermediate stages, which allows it to be stored in small portions in the form of high-energy compounds (ATP). The reduction of an oxygen atom when interacting with a pair of protons and electrons leads to the formation of a water molecule.
Tissue respiration
This is the process of consumption of oxygen by the cells of body tissues, which is involved in biological oxidation. This type of oxidation is called aerobic oxidation . If the final acceptor in the hydrogen transfer chain is not oxygen, but other substances (for example, pyruvic acid), then this type of oxidation is called anaerobic.
That. biological oxidation is the dehydrogenation of a substrate with the help of intermediate hydrogen carriers and its final acceptor.
Respiratory chain (tissue respiration enzymes) are carriers of protons and electrons from the oxidized substrate to oxygen. An oxidizing agent is a compound that can accept electrons. This ability is quantitatively characterized redox potential relative to a standard hydrogen electrode whose pH is 7.0. The lower the potential of a compound, the stronger its reducing properties and vice versa.
That. any compound can only donate electrons to a compound with a higher redox potential. In the respiratory chain, each subsequent link has a higher potential than the previous one.
The respiratory chain consists of:
1. NAD-dependent dehydrogenase;
2. FAD-dependent dehydrogenase;
3. Ubiquinone (Ko Q);
4. Cytochrome b, c, a + a 3.
NAD-dependent dehydrogenases . Contains as a coenzyme ABOVE And NADP. The pyridine ring of nicotinamide is capable of accepting electrons and hydrogen protons.
FAD and FMN-dependent dehydrogenases contain phosphorus ester of vitamin B 2 as a coenzyme ( FAD).
Ubiquinone (Ko Q ) takes away hydrogen from flavoproteins and turns into hydroquinone.
Cytochromes - chromoprotein proteins capable of acquiring electrons due to the presence of iron porphyrins as prosthetic groups in their composition. They accept an electron from a substance that is a slightly stronger reducing agent and transfer it to a stronger oxidizing agent. The iron atom is bonded to the nitrogen atom of the imidazole ring of the histidine amino acid on one side of the plane of the porphyrin ring, and on the other side to the sulfur atom of methionine. Therefore, the potential ability of the iron atom in cytochromes to bind oxygen is suppressed.
IN cytochrome c the porphyrin plane is covalently linked to the protein through two cysteine residues, and in cytochromexb And , it is not covalently bonded with protein.
IN cytochrome a+a 3 (cytochrome oxidase) instead of protoporphyrin contains porphyrin A, which differs in a number of structural features. The fifth coordination position of iron is occupied by an amino group belonging to an amino sugar residue that is part of the protein itself.
Unlike heme, hemolgobin, the iron atom in cytochromes can reversibly transform from a two to a trivalent state, which ensures electron transport (See Appendix 1 “Atomic and electronic structure of hemoproteins” for more details).
The mechanism of operation of the electron transport chain
The outer membrane of the mitochondrion (Fig. 4.8.1) is permeable to most small molecules and ions, the inner membrane is permeable to almost all ions (except H protons) and to most uncharged molecules.
All of the above components of the respiratory chain are embedded in the inner membrane. The transport of protons and electrons along the respiratory chain is ensured by the potential difference between its components. In this case, each increase in potential by 0.16 V releases energy sufficient for the synthesis of one ATP molecule from ADP and H 3 PO 4. When one molecule of O2 is consumed, 3 is formed ATP.
The processes of oxidation and formation of ATP from ADP and phosphoric acid i.e. Phosphorylation occurs in mitochondria. The inner membrane forms many folds - cristae. The space is bounded by an internal membrane - the matrix. The space between the inner and outer membranes is called intermembrane.
Such a molecule contains three high-energy bonds. Macroergic or energy-rich is a chemical bond that, when broken, releases more than 4 kcal/mol. The hydrolytic breakdown of ATP to ADP and phosphoric acid releases 7.3 kcal/mol. Exactly the same amount is spent to form ATP from ADP and phosphoric acid residue, and this is one of the main ways to store energy in the body.
During the transport of electrons along the respiratory chain, energy is released, which is spent on the addition of a phosphoric acid residue to ADP to form one molecule of ATP and one molecule of water. During the transfer of one pair of electrons along the respiratory chain, 21.3 kcal/mol is released and stored in the form of three ATP molecules. This accounts for about 40% of the energy released during electron transport.
This method of storing energy in a cell is called oxidative phosphorylation or coupled phosphorylation.
The molecular mechanisms of this process are most fully explained by Mitchell's chemoosmotic theory, put forward in 1961.
Mechanism of oxidative phosphorylation (Fig. 4.8.2.):
1) NAD-dependent dehydrogenase is located on the matrix surface of the inner mitochondrial membrane and donates a pair of hydrogen electrons to FMN-dependent dehydrogenase. In this case, a pair of protons also passes from the matrix to FMN and, as a result, FMN H 2 is formed. At this time, a pair of protons belonging to NAD is pushed into the intermembrane space.
2) FAD-dependent dehydrogenase donates a pair of electrons to Co Q and pushes a couple of protons into the intermembrane space. Having received electrons Co Q accepts a pair of protons from the matrix and turns into Co QH 2.
3) Ko Q H2 pushes a pair of protons into the intermembrane space, and a pair of electrons is transferred to cytochromes and then to oxygen to form a water molecule.
As a result, when a pair of electrons is transferred along a chain from the matrix to the intermembrane space, 6 protons (3 pairs) are pumped, which leads to the creation of a potential difference and a pH difference between the surfaces of the inner membrane.
4) The potential difference and pH difference ensure the movement of protons through the proton channel back into the matrix.
5) This reverse movement of protons leads to the activation of ATP synthase and the synthesis of ATP from ADP and phosphoric acid. When transferring one pair of electrons (i.e. three pairs of protons), 3 ATP molecules are synthesized (Fig. 4.7.3.).

Dissociation of the processes of respiration and oxidative phosphorylation occurs when protons begin to penetrate the inner membrane of mitochondria. In this case, the pH gradient is leveled and the driving force for phosphorylation disappears. Chemical uncouplers are called protonophores; they are capable of transporting protons across a membrane. These include 2,4-dinitrophenol, thyroid hormones, etc. (Fig. 4.8.3.).
The resulting ATP from the matrix into the cytoplasm is transferred by translocase enzymes, while in the opposite direction one molecule of ADP and one molecule of phosphoric acid are transferred into the matrix. It is clear that disruption of ADP and phosphate transport inhibits ATP synthesis.
The rate of oxidative phosphorylation depends primarily on the ATP content; the faster it is consumed, the more ADP accumulates, the greater the energy requirement and, therefore, the more active the process of oxidative phosphorylation. The regulation of the rate of oxidative phosphorylation by the cellular concentration of ADP is called respiratory control.

REFERENCES FOR THE CHAPTER IV.8.
1. Byshevsky A. Sh., Tersenov O. A. Biochemistry for the doctor // Ekaterinburg: Uralsky Rabochiy, 1994, 384 pp.;
2. Knorre D. G., Myzina S. D. Biological chemistry. – M.: Higher. school 1998, 479 pp.;
3. Leninger A. Biochemistry. Molecular basis of cell structure and functions // M.: Mir, 1974, 956 pp.;
4. Pustovalova L.M. Workshop on biochemistry // Rostov-on-Don: Phoenix, 1999, 540 pp.;
5. Stepanov V. M. Molecular biology. Structure and functions of proteins // M.: Higher school, 1996, 335 pp.;
The entire diversity of organisms living on Earth can be divided into two main groups, distinguished by the use of different energy sources - autotrophic and heterotrophic organisms. The first (autotrophs) are primarily green plants that are capable of directly using the radiant energy of the Sun in the process of photosynthesis, creating organic compounds (carbohydrates, amino acids, fatty acids, etc.) from inorganic ones. Other living organisms assimilate ready-made organic substances, using them as a source of energy or plastic material to build their bodies. It should be noted that most microorganisms are also heterotrophs. However, they are not able to absorb whole food particles. They secrete into their environment special digestive enzymes that break down food substances, turning them into small, soluble molecules, and these molecules penetrate into cells. As a result of metabolism, substances consumed with food are converted into the cell's own substances and structures and, in addition, the body is provided with energy to perform external work. Self-reproduction, i.e., constant renewal of body structures and reproduction, is the most characteristic feature of metabolism in living organisms, distinguishing it from metabolism in inanimate nature. |
Metabolism, inextricably linked with energy exchange, is a natural order of transformation of matter and energy in living systems, aimed at their preservation and self-reproduction. F. Engels noted metabolism as the most important property of life, with the cessation of which life itself ceases. He emphasized the dialectical nature of this process and pointed out that
From a consistently materialistic perspective, the founder of Russian physiology, I.M. Sechenov, considered the role of metabolism in the life of organisms. K. A. Timiryazev consistently pursued the idea that the main property that characterizes living organisms is a constant active exchange between the substance that makes up the organism and the substance of the environment, which the organism constantly perceives, assimilates, transforms it into something similar, again changes and distinguishes in the process of dissimilation. I.P. Pavlov considered metabolism as the basis for the manifestation of life activity, as the basis for the physiological functions of the body. A significant contribution to the knowledge of the chemistry of life processes was made by A.I. Oparin, who studied the basic patterns of the evolution of metabolism during the emergence and development of life on Earth.
BASIC CONCEPTS AND TERMS
Or metabolism is a set of chemical reactions in the body that provide it with the substances and energy necessary for life: self-preservation and self-reproduction. Self-reproduction is understood as the transformation of a substance coming from outside into the substances and structures of the body itself, resulting in continuous tissue renewal, growth and reproduction.
In metabolism there are:
- external exchange- includes the extracellular transformation of substances along the paths of their entry into the body and the removal of metabolic products from it [show]
.
The intake of substances into the body and the release of metabolic products together constitute the exchange of substances between the environment and the organism, and is defined as external exchange.
External exchange of substances (and energy) occurs constantly.
The human body from the external environment receives oxygen, water, mineral salts, nutrients, vitamins necessary for the construction and renewal of the structural elements of cells and tissues, and the formation of energy. All these substances can be called food products, some of which are of biological origin (plant and animal products) and a smaller part is non-biological (water and mineral salts dissolved in it).
Nutrients supplied with food undergo decomposition with the formation of amino acids, monosaccharides, fatty acids, nucleotides and other substances, which, when mixed with the same substances formed during the continuous breakdown of the structural and functional components of the cell, constitute the total pool of metabolites of the body. This fund is spent in two directions: part is used to renew the decayed structural and functional components of the cell; the other part is converted into end products of metabolism, which are excreted from the body.
When substances decompose into final metabolic products, energy is released; in an adult, 8,000-12,000 kJ (2,000-3,000 kcal) per day. This energy is used by the cells of the body to perform various types of work, as well as to maintain body temperature at a constant level.
- intermediate exchange- includes the transformation of substances inside biological cells from the moment they enter until the formation of final products (for example, amino acid metabolism, carbohydrate metabolism, etc.)
Metabolic stages. There are three successive stages.
Read more about
- intake (Nutrition is an integral part of metabolism (the intake of substances from the environment into the body))
- digestion (Biochemistry of digestion (digestion of nutrients))
- absorption (Biochemistry of digestion (absorption of nutrients))
II. Movements and transformations of substances in the body (intermediate metabolism) |
Intermediate metabolism (or metabolism) is the transformation of substances in the body from the moment they enter the cells until the formation of the final metabolic products, i.e., a set of chemical reactions that occur in living cells and provide the body with substances and energy for its vital activity, growth, and reproduction. This is the most complex part of metabolism.
Once inside the cell, the nutrient is metabolized - undergoes a series of chemical changes catalyzed by enzymes. The specific sequence of such chemical changes is called a metabolic pathway, and the resulting intermediate products are called metabolites. Metabolic pathways can be represented in the form of a metabolic map.
| Nutrient Metabolism | ||
| Carbohydrates | Lipids | Belkov |
Catabolic pathways of carbohydrates
Anabolic pathways of carbohydrates
|
Lipid catabolic pathway
Anabolic lipid pathway
|
Protein catabolic pathway
Anabolic pathway of amino acids
|
Due to competition between the phospholipid and triacylglycerol synthesis pathways for common substrates, all substances that promote phospholipid synthesis prevent the deposition of triacylglycerols in tissues. These substances are called lipotropic factors. These include structures and components of phospholipids: choline, inositol, serine; a substance that facilitates the decarboxylation of serine phosphatides - pyridoxal phosphate; methyl group donor - methionine; folic acid and cyanocobalamin, involved in the formation of methyl group transfer coenzymes (THFA and methylcobalamin). They can be used as medications that prevent excessive deposition of triacylglycerol in tissues (fatty infiltration).
|
||
| Table 24. Human daily metabolism (rounded values; adult with body weight about 70 kg) | |||
| Substances | Content in the body, g | Daily consumption, g | Daily allocation |
| O2 | - | 850 | - |
| CO2 | - | - | 1000 |
| Water | 42 000 | 2200 | 2600 |
| Organic matter: | |||
| squirrels | 15 000 | 80 | - |
| lipids | 10 000 | 100 | - |
| carbohydrates | 700 | 400 | - |
| nucleic acids | 700 | - | - |
| urea | - | - | 30 |
| Mineral salts | 3 500 | 20 | 20 |
| Total | 71 900 | 3650 | 3650 |
As a result of metabolic activity, harmful substances are formed in all parts of the body, which enter the blood and must be removed. This function is performed by the kidneys, which separate harmful substances and send them to the bladder, from where they are then excreted from the body. Other organs also take part in the metabolic process: liver, pancreas, gall bladder, intestines, sweat glands.
A person excretes the main end products of metabolism in urine, feces, sweat, and exhaled air - CO 2, H 2 O, urea H 2 N - CO - NH 2. Hydrogen of organic substances is excreted in the form of H 2 O, and the body releases more water than it consumes (see Table 24): approximately 400 g of water is formed per day in the body from hydrogen of organic substances and oxygen of inhaled air (metabolic water). Carbon and oxygen of organic substances are removed in the form of CO 2, and nitrogen is removed in the form of urea.
In addition, a person secretes a lot of other substances, but in small quantities, so that their contribution to the overall balance of metabolism between the body and the environment is small. However, it should be noted that the physiological significance of the release of such substances can be significant. For example, disruption of the release of heme breakdown products or metabolic products of foreign compounds, including drugs, can cause severe metabolic disorders and body functions.
Metabolic substrates- chemical compounds coming from food. Among them, two groups can be distinguished: main nutritional substances (carbohydrates, proteins, lipids) and minor ones, supplied in small quantities (vitamins, mineral compounds).
It is customary to distinguish between replaceable and irreplaceable nutrients. Essential nutrients are those that cannot be synthesized in the body and, therefore, must be supplied with food.
Metabolic pathway- this is the nature and sequence of chemical transformations of a specific substance in the body. The intermediate products formed during the transformation process are called metabolites, and the last compound of the metabolic pathway is the final product.
Chemical transformations occur continuously in the body. As a result of the body's nutrition, the starting substances undergo metabolic transformations; The end products of metabolism are constantly removed from the body. Thus, the organism is a thermodynamically open chemical system. The simplest example of a metabolic system is a single unbranched metabolic chain:




-->a -->b -->c -->d -->
With a constant flow of substances in such a system, a dynamic equilibrium is established when the rate of formation of each metabolite is equal to the rate of its consumption. This means that the concentration of each metabolite remains constant. This state of the system is called stationary, and the concentrations of substances in this state are called stationary concentrations.
A living organism at any given moment does not meet the given definition of a stationary state. However, considering the average value of its parameters over a relatively large period of time, one can note their relative constancy and thereby justify the application of the concept of a stationary system to living organisms [show] .

In Fig. 64 presents a hydrodynamic model of an unbranched metabolic chain. In this device, the height of the liquid column in the cylinders models the concentrations of metabolites a-d, respectively, and the throughput of the connecting tubes between the cylinders models the rate of the corresponding enzymatic reactions.
At a constant rate of liquid entering the system, the height of the liquid column in all cylinders remains constant: this is a stationary state.
If the rate of fluid entry increases, then the height of the liquid column in all cylinders and the rate of fluid flow through the entire system will increase: the system has moved to a new stationary state. Similar transitions occur in metabolic processes in a living cell.
Regulation of metabolite concentrations
Typically, there is a reaction in a metabolic chain that proceeds much slower than all other reactions - this is the rate-limiting step in the pathway. In the figure, such a stage is modeled by a narrow connecting tube between the first and second cylinders. The rate-limiting stage determines the overall rate of conversion of the starting substance into the final product of the metabolic chain. Often the enzyme that catalyzes the rate-limiting reaction is a regulatory enzyme: its activity can change under the influence of cellular inhibitors and activators. In this way, regulation of the metabolic pathway is ensured. In Fig. 64, a transition tube with a valve between the first and second cylinders models a regulatory enzyme: by raising or lowering the valve, the system can be transferred to a new stationary state, with a different overall fluid flow rate and other fluid levels in the cylinders.
In branched metabolic systems, regulatory enzymes usually catalyze the first reactions at the branch site, such as reactions b --> c and b --> i in Fig. 65. This ensures the possibility of independent regulation of each branch of the metabolic system.

Many metabolic reactions are reversible; the direction of their flow in a living cell is determined by the consumption of the product in a subsequent reaction or the removal of the product from the reaction sphere, for example, by excretion (Fig. 65).
When the state of the body changes (food intake, transition from rest to physical activity, etc.), the concentration of metabolites in the body changes, i.e., a new stationary state is established. However, under the same conditions, for example, after a night's sleep (before breakfast), they are approximately the same in all healthy people; Due to the action of regulatory mechanisms, the concentration of each metabolite is maintained at its characteristic level. The average values of these concentrations (indicating the limits of fluctuations) serve as one of the characteristics of the norm. In diseases, the steady-state concentrations of metabolites change, and these changes are often specific to a particular disease. Many biochemical methods for laboratory diagnosis of diseases are based on this.
There are two directions in the metabolic pathway - anabolism and catabolism (Fig. 1).
- Anabolic reactions are aimed at converting simpler substances into more complex ones that form the structural and functional components of the cell, such as coenzymes, hormones, proteins, nucleic acids, etc. These reactions are predominantly reductive, accompanied by the expenditure of free chemical energy (endergonic reactions). The source of energy for them is the process of catabolism. In addition, catabolic energy is used to ensure the functional activity of the cell (motor and others).
- Catabolic transformations are the processes of breakdown of complex molecules, both those received with food and those included in the cell, into simple components (carbon dioxide and water); these reactions are usually oxidative and are accompanied by the release of free energy (exergonic reactions).
Amphibolic pathway(dual) - a path during which catabolic and anabolic transformations are combined, i.e. Along with the destruction of one compound, the synthesis of another occurs.
Amphibolic pathways are associated with the terminal, or final, oxidation system of substances, where they burn to final products (CO 2 and H 2 O) with the formation of large amounts of energy. In addition to them, the final products of metabolism are urea and uric acid, which are formed in special reactions of the exchange of amino acids and nucleotides. The connection between metabolism through the ATP-ADP system and the amphibolic cycle of metabolites is shown schematically in Fig. 2.
ATP-ADP system(ATP-ADP cycle) is a cycle in which the continuous formation of ATP molecules occurs, the hydrolysis energy of which is used by the body in various types of work.
This is a metabolic pathway in which one of the end products is identical to one of the compounds involved in this process (Fig. 3).
Anaplerotic pathway- metabolic, the final product of which is identical to one of the intermediate products of any cyclic pathway. The anaplerotic pathway in the example of Fig. 3 replenishes the cycle with product X (anaplerosis - replenishment).
Let's use this example. Buses of brands X, Y, Z operate in the city. Their routes are shown in the diagram (Fig. 4).
Based on this example, we define the following.
- A particular metabolic pathway is a set of transformations characteristic only of a specific compound (for example, carbohydrates, lipids or amino acids).
- The general metabolic pathway is a set of transformations that involve two or more types of compounds (for example, carbohydrates and lipids or carbohydrates, lipids and amino acids).
Localization of metabolic pathways
Catabolic and anabolic pathways in eukaryotic individuals differ in their localization in the cell (Table 22.).
This division is due to the confinement of enzyme systems to certain areas of the cell (compartmentalization), which ensures both segregation and integration of intracellular functions, as well as appropriate control.
Currently, thanks to electron microscopic and histochemical studies, as well as the method of differential centrifugation, significant progress has been made in determining the intracellular localization of enzymes. As can be seen from Fig. 74, in a cell you can find a cellular, or plasma, membrane, nucleus, mitochondria, lysosomes, ribosomes, a system of tubules and vesicles - endoplasmic reticulum, lamellar complex, various vacuoles, intracellular inclusions, etc. The main undifferentiated part of the cell cytoplasm in terms of mass is hyaloplasm ( or cytosol).
It has been established that RNA polymerases, i.e., enzymes that catalyze the formation of mRNA, are localized in the nucleus (more precisely, in the nucleolus). The nucleus contains enzymes involved in the process of DNA replication and some others (Table 23).

| Table 23. Localization of some enzymes inside the cell | |
| Cytosol | Glycolytic enzymes Pentose pathway enzymes Amino acid activation enzymes Fatty acid synthesis enzymes Phosphorylase Glycogen synthase |
| Mitochondria | Pyruvate dehydrogenase complex Krebs cycle enzymes Enzymes of the fatty acid oxidation cycle Enzymes of biological oxidation and oxidative phosphorylation |
| Lysosomes | Acid hydrolases |
| Microsomal fraction | Ribosomal enzymes of protein synthesis Enzymes for the synthesis of phospholipids, triglycerides, as well as a number of enzymes involved in the synthesis of cholesterol Hydroxylases |
| Plasma membrane | Adenylate cyclase, Na+-K+-dependent ATPase |
| Core | Enzymes involved in the process of DNA replication RNA polymerase NAD synthetase |
Relationship between enzymes and cell structures:
- Mitochondria. Enzymes of the chain of biological oxidation (tissue respiration) and oxidative phosphorylation, as well as enzymes of the pyruvate dehydrogenase complex, the tricarboxylic acid cycle, urea synthesis, fatty acid oxidation, etc. are associated with mitochondria.
- Lysosomes. Lysosomes contain mainly hydrolytic enzymes with an optimum pH in the region of 5. It is because of the hydrolytic nature of the enzymes that these particles are called lysosomes.
- Ribosomes. Enzymes of protein synthesis are localized in ribosomes; in these particles, mRNA is translated and amino acids are linked into polypeptide chains to form protein molecules.
- Endoplasmic reticulum. The endoplasmic reticulum contains enzymes for lipid synthesis, as well as enzymes involved in hydroxylation reactions.
- Plasma membrane. The plasma membrane is primarily associated with ATPase, which transports Na + and K + , adenylate cyclase and a number of other enzymes.
- Cytosol. The cytosol (hyaloplasm) contains enzymes of glycolysis, the pentose cycle, the synthesis of fatty acids and mononucleotides, the activation of amino acids, as well as many enzymes of gluconeogenesis.
In table 23 summarizes data on the localization of the most important enzymes and individual metabolic stages in various subcellular structures.
Multienzyme systems are localized in the structure of organelles in such a way that each enzyme is located in close proximity to the next enzyme in a given sequence of reactions. Due to this, the time required for the diffusion of reaction intermediates is reduced, and the entire sequence of reactions is strictly coordinated in time and space. This is true, for example, for enzymes involved in the oxidation of pyruvic acid and fatty acids, in protein synthesis, as well as for enzymes of electron transfer and oxidative phosphorylation.
Compartmentalization also ensures that chemically incompatible reactions occur at the same time, i.e. independence of the paths of catabolism and anabolism. Thus, in a cell, the oxidation of long-chain fatty acids to the acetyl-CoA stage and the opposite process, the synthesis of fatty acids from acetyl-CoA, can simultaneously occur. These chemically incompatible processes occur in different parts of the cell: the oxidation of fatty acids in the mitochondria, and their synthesis outside the mitochondria in the hyaloplasm. If these paths coincided and differed only in the direction of the process, then so-called useless, or futile, cycles would arise in the exchange. Such cycles occur in pathology, when useless circulation of metabolites is possible.
The elucidation of individual links of metabolism in different classes of plants, animals and microorganisms reveals a fundamental commonality of the paths of biochemical transformations in living nature.
BASIC PROVISIONS OF METABOLISM REGULATION
Regulation of metabolism at the cellular and subcellular levels is carried out
- by regulating the synthesis and catalytic activity of enzymes.
Such regulatory mechanisms include
- suppression of enzyme synthesis by end products of the metabolic pathway,
- induction of the synthesis of one or more enzymes by substrates,
- modulation of the activity of already present enzyme molecules,
- regulation of the rate of entry of metabolites into the cell. Here the leading role is played by the biological membranes surrounding the protoplasm and the nucleus, mitochondria, lysosomes and other subcellular organelles located in it.
- by regulating the synthesis and activity of hormones. Thus, protein metabolism is influenced by the thyroid hormone - thyroxine; fat metabolism is influenced by the hormones of the pancreas and thyroid glands, adrenal glands and pituitary gland; carbohydrate metabolism is influenced by the hormones of the pancreas (insulin) and adrenal glands (adrenaline). A special role in the mechanism of action of hormones belongs to cyclic nucleotides (cAMP and cGMP).
In animals and humans, hormonal regulation of metabolism is closely related to the coordinating activity of the nervous system. An example of the influence of the nervous system on carbohydrate metabolism is the so-called sugar injection of Claude Bernard, which leads to hyperglycemia and glycosuria.
- The most important role in the processes of metabolic integration belongs to the cerebral cortex. As I. P. Pavlov pointed out: “The more perfect the nervous system of an animal organism, the more centralized it is, the higher its department is more and more the manager and distributor of all the activities of the organism... This higher department contains under its jurisdiction all the phenomena occurring in body".
Thus, a special combination, strict coordination and rate of metabolic reactions together form a system that reveals the properties of a feedback mechanism (positive or negative).

METHODS FOR STUDYING INTERMEDIATE METABOLISM
Two approaches are used to study metabolism:
- studies on the whole organism (in vivo experiments) [show]
A classic example of research on a whole organism, carried out at the beginning of this century, is the Knoop experiments. He studied the way fatty acids break down in the body. To do this, Knoop fed dogs various fatty acids with an even (I) and odd (II) number of carbon atoms, in which one hydrogen atom in the methyl group was replaced by a phenyl radical C6H5:
In the first case, phenylacetic acid C 6 H 5 -CH 2 -COOH was always excreted in the urine of dogs, and in the second - benzoic acid C 6 H 5 -COOH. Based on these results, Knoop concluded that the breakdown of fatty acids in the body occurs through the sequential elimination of two-carbon fragments, starting from the carboxyl end:
CH 3 -CH 2 -|-CH 2 -CH 2 -|-CH 2 -CH 2 -|-CH 2 -CH 2 -|-CH 2 - COOH
This conclusion was later confirmed by other methods.
Essentially, in these studies, Knoop used the method of labeling molecules: he used a phenyl radical, which does not undergo changes in the body, as a label. Starting around the 40s of the XX century. The use of substances whose molecules contain radioactive or heavy isotopes of elements has become widespread. For example, by feeding experimental animals various compounds containing radioactive carbon (14 C), it was established that all the carbon atoms in the cholesterol molecule come from the carbon atoms of acetate:

Typically, either stable isotopes of elements that differ in mass from elements commonly found in the body (usually heavy isotopes) or radioactive isotopes are used. Of the stable isotopes, the most commonly used isotopes are hydrogen with a mass of 2 (deuterium, 2 H), nitrogen with a mass of 15 (15 N), carbon with a mass of 13 (13 C) and oxygen with a mass of 18 (18 C). Of the radioactive isotopes, the isotopes of hydrogen (tritium, 3 H), phosphorus (32 P and 33 P), carbon (14 C), sulfur (35 S), iodine (131 I), iron (59 Fe), sodium (54 Na) are used ) and etc.
Having labeled a molecule of the compound under study using a stable or radioactive isotope and introduced it into the body, the labeled atoms or chemical groups containing them are then determined and, having discovered them in certain compounds, a conclusion is made about the ways in which the labeled substance is transformed in the body. Using an isotope label, you can also determine the residence time of a substance in the body, which, to a certain approximation, characterizes the biological half-life, i.e., the time during which the amount of an isotope or labeled compound is halved, or obtain accurate information regarding the permeability of the membranes of individual cells. Isotopes are also used to determine whether a given substance is a precursor or breakdown product of another compound, and to determine the rate of tissue turnover. Finally, when several metabolic pathways exist, it is possible to determine which one is dominant.
In studies on whole organisms, the body's nutritional requirements are also studied: if the elimination of a substance from the diet leads to disruption of growth and development or physiological functions of the body, then this substance is an essential nutritional factor. The required amounts of nutrients are determined in a similar way.
- and studies on isolated parts of the body - analytical-disintegrating methods (in vitro experiments, i.e. outside the body, in a test tube or other laboratory vessels). The principle of these methods is the gradual simplification, or rather disintegration, of a complex biological system in order to isolate individual processes. If we consider these methods in descending order, that is, from more complex to simpler systems, then they can be arranged in the following order:
- removal of individual organs [show]
When organs are removed, there are two objects of study: an organism without a removed organ and an isolated organ.
Isolated organs. If a solution of a substance is injected into the artery of an isolated organ and the substances are analyzed in the liquid flowing from the vein, then it is possible to establish what transformations this substance undergoes in the organ. For example, in this way it was found that the liver serves as the main site of formation of ketone bodies and urea.
Similar experiments can be carried out on organs without isolating them from the body (arteriovenous difference method): in these cases, blood for analysis is taken using cannulas inserted into the artery and vein of the organ, or using a syringe. In this way, for example, it can be established that in the blood flowing from working muscles, the concentration of lactic acid is increased, and when flowing through the liver, the blood is freed from lactic acid.
- tissue section method [show]
Sections are thin pieces of tissue that are made using a microtome or simply a razor blade. The sections are incubated in a solution containing nutrients (glucose or others) and a substance whose transformations in cells of a given type are wanted to be determined. After incubation, the metabolic products of the test substance in the incubation liquid are analyzed.
The method of tissue sections was first proposed by Warburg in the early 20s. Using this technique, it is possible to study tissue respiration (oxygen consumption and carbon dioxide release by tissues). A significant limitation in the study of metabolism in the case of using tissue sections are cell membranes, which often act as barriers between the contents of the cell and the “nutrient” solution.
- homogenates and subcellular fractions [show]

Homogenates are cell-free preparations. They are obtained by destroying cell membranes by rubbing fabric with sand or in special devices - homogenizers (Fig. 66). In homogenates there is no impermeability barrier between the added substrates and the enzymes.
The destruction of cell membranes allows direct contact between the cell contents and the added compounds. This makes it possible to establish which enzymes, coenzymes and substrates are important for the process under study.
Fractionation of homogenates. From the homogenate, subcellular particles can be isolated, both supramolecular (cellular organelles) and individual compounds (enzymes and other proteins, nucleic acids, metabolites). For example, using differential centrifugation, you can obtain fractions of nuclei, mitochondria, and microsomes (microsomes are fragments of the endoplasmic reticulum). These organelles vary in size and density and are therefore sedimented at different centrifugation speeds. The use of isolated organelles makes it possible to study the metabolic processes associated with them. For example, isolated ribosomes are used to study the pathways and mechanisms of protein synthesis, and mitochondria are used to study the oxidative reactions of the Krebs cycle or the chain of respiratory enzymes.
After sedimentation of microsomes, soluble components of the cell remain in the supernatant - soluble proteins, metabolites. Each of these fractions can be further fractionated using different methods, isolating their constituent components. From the isolated components, it is possible to reconstruct biochemical systems, for example, a simple “enzyme + substrate” system and such complex ones as systems for the synthesis of proteins and nucleic acids.
- partial or complete reconstruction of an enzyme system in vitro using enzymes, coenzymes and other reaction components [show]
Used to integrate highly purified enzymes and coenzymes. For example, using this method it became possible to completely reproduce a fermentation system that has all the essential features of yeast fermentation.
- removal of individual organs [show]
Of course, these methods are of value only as a step necessary to achieve the ultimate goal - understanding the functioning of the whole organism.
FEATURES OF STUDYING HUMAN BIOCHEMISTRY
There are far-reaching similarities in the molecular processes of the different organisms that inhabit the Earth. Such fundamental processes as matrix biosynthesis, mechanisms of energy transformation, and the main pathways of metabolic transformations of substances are approximately the same in organisms from bacteria to higher animals. Therefore, many of the results of studies conducted with E. coli appear to be applicable to humans. The greater the phylogenetic relatedness of species, the more common their molecular processes are.
The overwhelming majority of knowledge about human biochemistry is obtained in this way: based on known biochemical processes in other animals, a hypothesis is built about the most likely version of this process in the human body, and then the hypothesis is tested by direct studies of human cells and tissues. This approach makes it possible to conduct research on a small amount of biological material obtained from humans. The most commonly used tissues are tissues removed during surgical operations, blood cells (erythrocytes and leukocytes), as well as human tissue cells grown in culture in vitro.
The study of hereditary human diseases, necessary for the development of effective methods for their treatment, simultaneously provides a lot of information about the biochemical processes in the human body. In particular, a congenital defect of the enzyme causes its substrate to accumulate in the body; when studying such metabolic disorders, sometimes new enzymes and reactions are discovered, quantitatively insignificant (which is why they were not noticed when studying the norm), which, however, have vital significance.
Modular unit 1 ENZYMES AS PROTEIN CATALYSTS
Learning objectives Be able to:
1. Explain the properties of enzymes and the features of enzymatic catalysis by their protein nature.
2. Assess the role of vitamins in human nutrition as substrates for the synthesis of coenzymes.
3. Determine whether enzymes belong to a certain class and subclass in accordance with their nomenclature.
4. Calculate enzyme activity and evaluate the affinity of the enzyme for the substrate.
Know:
1. Structural features of enzymes as protein catalysts.
2. Types of enzyme specificity.
3. Basics of classification of enzymes, classes of enzymes, examples of reactions catalyzed by enzymes.
4. The structure of coenzymes and cofactors and their role in enzymatic catalysis, the role of vitamins in this process.
5. Basics of enzymatic kinetics.
6. Units of enzyme activity and methods for their determination.
TOPIC 2.1. PROPERTIES OF ENZYME AS PROTEIN
CATALYST
1. Enzymes are protein catalysts accelerating chemical reactions in living cells. They have all the properties characteristic of proteins and certain structural features that determine their catalytic properties. Enzymes, in addition, obey the general laws of catalysis and have properties characteristic of non-biological catalysts: they accelerate energetically possible reactions, keep the energy of the chemical system constant, and are not consumed during the reaction process.
2. Enzymes are characterized by:
specificity. The biological function of an enzyme, like any protein, is determined by the presence in its structure of an active center with which a specific ligand interacts. The ligand that interacts with the active site of the enzyme is called substrate.
catalytic efficiency. Most enzyme-catalyzed reactions are highly efficient, occurring 10 8 -10 14 times faster than non-catalyzed reactions. Each enzyme molecule is capable of transforming from 100 to 1000 molecules of substrate into a product per second.
conformational lability. The catalytic efficiency of an enzyme, like any protein molecule, depends on its conformation and, in particular, on the conformation of the active center. There are substances in cells that can cause minor changes in the conformation of the enzyme molecule due to the breaking of some and the formation of other weak bonds; this can cause either an increase or decrease in enzyme activity.
3. Enzyme activity can be regulated. The action of enzymes in a cell, as a rule, is strictly ordered: the product of one enzymatic reaction is the substrate of another; thus forming metabolic pathways. Among the many enzymes of almost every metabolic pathway, there are key, or regulatory, enzymes whose activity can vary depending on the cell's need for the final product of the metabolic pathway.
4. Optimal conditions for enzymatic reactions: temperature 37-38 °C; normal atmospheric pressure, pH 6.9-7.7, characteristic of most tissues. In contrast, efficient chemical catalysis often requires high temperatures and pressures, as well as extreme pH values.
TOPIC 2.2. ACTIVE CENTER: SPECIFICITY OF ENZYME ACTION
1. Active site of enzymes- this is a certain section of a protein molecule that is capable of complementarily binding to the substrate and ensuring its catalytic transformation. The structure of the active center is formed by amino acid radicals, just as in the case of the active center of any protein. The active center of the enzyme contains amino acid residues, the functional groups of which ensure complementary binding of the substrate (binding site), and amino acid residues, the functional groups of which carry out the chemical transformation of the substrate (catalytic site) (Fig. 2.1).
Rice. 2.1. Scheme of the structure of the active center of the enzyme.
The amino acids that form the active center of the enzyme are marked in red: 1 - binding site; 2 - catalytic section
2. Specificity- the most important property of enzymes that determines the biological significance of enzymes. Distinguish substrate And catalytic specificity of the enzyme, which are determined by the structure of the active center.
3. Under substrate specificity refers to the ability of each enzyme to interact with only one or several specific substrates.
There are:
- absolute substrate specificity, if the active site of the enzyme is complementary to only one substrate;
- group substrate specificity, if the enzyme catalyzes the same type of reaction with a small amount (group) of structurally similar substrates;
- stereospecificity, if the enzyme exhibits absolute specificity for only one of the existing stereoisomers of the substrate.
4. Catalytic specificity or the specificity of the substrate conversion pathway, ensures the transformation of the same substrate under the action of different enzymes. This is ensured by the structure of the catalytic sites of the active centers of the corresponding enzymes. For example, a molecule
Glucose-6-phosphate in human liver cells is a substrate of four different enzymes: phosphoglucomutase, glucose-6-phosphate phosphatase, phosphoglucoisomerase and glucose-6-phosphate dehydrogenase. However, due to the structural features of the catalytic sites of these enzymes, various transformations of glucose-6-phosphate occur with the formation of four different products (Fig. 2.2).
 Rice. 2.2. Catalytic pathways for the conversion of glucose-6-phosphate.
Rice. 2.2. Catalytic pathways for the conversion of glucose-6-phosphate.
The specificity of the substrate conversion pathway makes it possible to transform the same substrate under the action of different enzymes. The glucose-6-phosphate molecule is a substrate for different enzymes, which leads to the formation of different products
TOPIC 2.3. MECHANISM OF ENZYME ACTION
1. During catalysis, the substrate, bound to the active site of the enzyme in an enzyme-substrate (ES) complex, undergoes a chemical conversion to a product, which is then released. The catalysis process can be schematically represented as follows:
The process of enzymatic catalysis can be divided into stages (Fig. 2.3). At stage I, the substrate approaches and orientations in the region of the active center of the enzyme. At stage II, as a result induced correspondence[change in the conformation of the substrate (S) and the active center of the enzyme] an enzyme-substrate complex (ES) is formed. At stage III, the bonds in the substrate are destabilized and an unstable enzyme-product complex (EP) is formed. At stage IV, the complex (EP) disintegrates with the release of reaction products from the active site and the release of the enzyme.
2. To understand the energy of a chemical reaction, it is necessary to take into account the change in the energy of substrates and reaction products, as well as the role of enzymes in this process. It is known that in order for a reaction to take place, substrates must receive such an amount of additional energy (called activation energy E a) that is necessary for the substrate molecules to enter into the reaction (Fig. 2.4). In the case of an enzymatic reaction, the activation energy decreases, which ensures a more efficient reaction.
 Rice. 2.3. Stages of enzymatic catalysis:
Rice. 2.3. Stages of enzymatic catalysis:
I - stage of approach and orientation of the substrate in the active center of the enzyme; II - formation of an enzyme-substrate complex (Eb); III - formation of an unstable enzyme-product complex (EP); IV - release of reaction products from the active center of the enzyme
 Rice. 2.4. Change in free energy during a chemical reaction, uncatalyzed and catalyzed by enzymes.
Rice. 2.4. Change in free energy during a chemical reaction, uncatalyzed and catalyzed by enzymes.
The enzyme reduces the activation energy E a, i.e. reduces the height of the energy barrier; as a result, the proportion of reactive molecules increases and the reaction rate increases
TOPIC 2.4. COFACTORS AND COENZYMS
Most enzymes require the presence of certain non-protein substances - cofactors - to exhibit catalytic activity. There are two groups of cofactors: metal ions and coenzymes.
1. Metal ions participate in the functioning of the enzyme in various ways.
Change the conformation of the substrate molecule, which ensures complementary interaction with the active center. For example, the Mg2+-ATP complex acts as a substrate.
Provide the native conformation of the active center of the enzyme. Ions
Mg 2 +, Mn 2 +, Zn 2 +, Co 2 +, Mo 2 + are involved in stabilizing the active center of enzymes and contribute to the addition of the coenzyme.
They stabilize the conformation of the enzyme protein molecule. For example, zinc ions are required to stabilize the quaternary structure of the enzyme alcohol dehydrogenase, which catalyzes the oxidation of ethanol.
Directly involved in enzymatic catalysis. Ions Zn 2 +, Fe 2 +, Mn 2 +, Cu 2 + take part in electrophilic catalysis. Metal ions with variable valence can also participate in electron transfer. For example, in cytochromes (heme-containing proteins), the iron ion is capable of attaching and donating one electron. Due to this property, cytochromes participate in redox reactions:
 2. Coenzymes are organic substances, most often derivatives of vitamins, that are directly involved in enzymatic catalysis, as they are located in the active center of enzymes. An enzyme containing a coenzyme and having enzymatic activity is called holoenzyme. The protein part of such an enzyme is called apoenzyme, which in the absence of a coenzyme has no catalytic activity.
2. Coenzymes are organic substances, most often derivatives of vitamins, that are directly involved in enzymatic catalysis, as they are located in the active center of enzymes. An enzyme containing a coenzyme and having enzymatic activity is called holoenzyme. The protein part of such an enzyme is called apoenzyme, which in the absence of a coenzyme has no catalytic activity.
 The coenzyme can bind to the protein part of the enzyme only at the time of the reaction or be associated with the apoenzyme by strong covalent bonds. In the latter case it is called prosthetic group. Examples of the most common coenzymes - vitamin derivatives, as well as their participation in enzymatic processes - are given in Table. 2.1.
The coenzyme can bind to the protein part of the enzyme only at the time of the reaction or be associated with the apoenzyme by strong covalent bonds. In the latter case it is called prosthetic group. Examples of the most common coenzymes - vitamin derivatives, as well as their participation in enzymatic processes - are given in Table. 2.1.
Table 2.1. Structure and function of major coenzymes
 End of table. 2.1.
End of table. 2.1.
 TOPIC 2.5. CLASSIFICATION AND NOMENCLATURE
TOPIC 2.5. CLASSIFICATION AND NOMENCLATURE
ENZYMES
1. The name of most enzymes contains the suffix "ase" attached to the name of the substrate of the reaction (for example: urease, sucrase, lipase, nuclease) or to the name of the chemical transformation of a particular substrate (for example: lactate dehydrogenase, adenylate cyclase, phosphoglucomutase, pyruvate carboxylase). However, a number of trivial, historically fixed names of enzymes have remained in use, which do not provide any information about the substrate or the type of chemical transformation (for example, trypsin, pepsin, renin, thrombin, etc.).
2. In order to systematize the enzymes found in nature, the International Union of Biochemistry and Molecular Biology (IUBMB) developed a nomenclature in 1961, according to which all enzymes are divided into six main classes depending on the type of chemical reaction they catalyze. Each class consists of numerous subclasses and subsubclasses, depending on the chemical group of the substrate being converted, the donor and acceptor of the converted groups, the presence of additional molecules, etc. Each of the six classes has its own serial number, strictly assigned to it: 1st class - oxidoreductases; 2nd class - transferases; 3rd grade - hydrolases; 4th grade - lyases; 5th grade - isomerases; 6th grade - ligases
This classification is necessary to accurately identify the enzyme: for each enzyme there is a code number. For example, the enzyme maldehydrogenase has the systematic name L-malate: NAD oxidoreductase and the code number is 1.1.1.38. The first digit indicates the enzyme class number (in this case, the number 1 indicates that the enzyme belongs to the class of oxidoreductases); the second digit indicates the type of reaction being catalyzed (in this example, the hydroxyl group is oxidized); the third digit means the presence of a coenzyme (in this case, the NAD+ coenzyme), the last digit is the serial number of the enzyme in this subgroup.
3. Characteristics of the main classes of enzymes with examples of the reactions they catalyze.
1. Oxidoreductases catalyze various redox reactions. The class is divided into subclasses:
A) dehydrogenases catalyze dehydrogenation reactions (elimination of hydrogen with the transfer of electrons from the dehydrogenated substrate to another acceptor). The coenzymes NAD+, NADP+, FAD, FMN are used as electron acceptors. This subclass includes the enzymes malate dehydrogenase (Fig. 2.5), isocitrate dehydrogenase, succinate dehydrogenase, α-ketobutyrate dehydrogenase, etc.;
 Rice. 2.5. Malate dehydrogenation reaction
Rice. 2.5. Malate dehydrogenation reaction
b) oxidases- catalyze oxidation reactions with the participation of molecular oxygen (Fig. 2.6);
Rice. 2.6. Reaction catalyzed by the enzyme cytochrome oxidase
V) oxygenases(hydroxylases) catalyze oxidation reactions by incorporating an oxygen atom into the hydroxyl group of the substrate molecule. The reaction occurs with the participation of molecular oxygen, one atom of which is attached to the substrate, and the second is involved in the formation of a water molecule (Fig. 2.7).
 Rice. 2.7. Hydroxylation reaction of phenylalanine.
Rice. 2.7. Hydroxylation reaction of phenylalanine.
Reaction coenzymes: tetrahydrobiopterin (H 4 BP) and dihydrobiopterin (H 2 BP)
2. Transferases- catalyze functional group transfer reactions. Depending on the transferred group, they are divided into subclasses: aminotransferases (Fig. 2.8), acyltransferases, methyltransferases, glycosyltransferases, kinases (phosphotransferases) (Fig. 2.9).
 Rice. 2.8. A reaction catalyzed by the enzyme ALT (Alanine-a-ketoglutarate aminotransferase), which belongs to the class of transferases, a subclass of aminotransferases.
Rice. 2.8. A reaction catalyzed by the enzyme ALT (Alanine-a-ketoglutarate aminotransferase), which belongs to the class of transferases, a subclass of aminotransferases.
PF - coenzyme pyridoxal phosphate
Rice. 2.9. A reaction catalyzed by the enzyme protein kinase, which belongs to the class of transferases, a subclass of phosphotransferases.
ATP is the donor of the phosphoric acid residue
3. Hydrolases catalyze hydrolysis reactions (cleavage of a covalent bond with the addition of a water molecule at the site of the break). They are divided into subclasses depending on the substrate. The names are formed depending on the substrate molecule or the specific chemical bond being hydrolyzed: protease, amylase, glycosidase, nuclease, esterase, phosphatase, etc. An example of a reaction scheme for the hydrolysis of a protein molecule is shown in Fig. 2.10.
Rice. 2.10. Protein molecule hydrolysis reaction
4. Lyases- lyases include enzymes that cleave certain groups from substrates in a non-hydrolytic way, such as CO 2, H 2 O, NH 2 SH 2, etc., or attach (for example, a water molecule) via a double bond. The decarboxylation reaction (elimination of a CO 2 molecule) is shown in Fig. 2.11, and the reaction of adding a water molecule (hydratase reaction) is in Fig. 2.12.
 Rice. 2.11. Decarboxylation reaction (elimination of a CO 2 molecule)
Rice. 2.11. Decarboxylation reaction (elimination of a CO 2 molecule)
PF coenzyme pyridoxal phosphate
 Rice. 2.12. The reaction of adding a water molecule to fumarate
Rice. 2.12. The reaction of adding a water molecule to fumarate
5. Isomerases catalyze various intramolecular transformations (Fig. 2.13).
 Rice. 2.13. Reaction catalyzed by the enzyme phosphoglucoisomerase
Rice. 2.13. Reaction catalyzed by the enzyme phosphoglucoisomerase
6. Ligases(synthetases) catalyze reactions that complicate a molecule by attaching two molecules to each other to form a covalent bond; in this case, the energy of ATP or other high-energy compounds is used (Fig. 2.14).
 Rice. 2.14. Reaction catalyzed by the enzyme glutamine synthetase
Rice. 2.14. Reaction catalyzed by the enzyme glutamine synthetase
TOPIC 2.6. BASICS OF ENZYMATIVE KINETICS
CATALYSIS
1. The kinetics of enzymatic reactions is a branch of enzymology that studies the dependence of the rate of chemical reactions catalyzed by enzymes on the chemical nature of the reacting substances and environmental factors.
To measure the catalytic activity of enzymes, indicators such as reaction rate or enzyme activity are used. Enzyme reaction rate determined by a decrease in the number of substrate molecules or an increase in the number of product molecules per unit time. The rate of an enzymatic reaction is a measure of the catalytic activity of the enzyme and is denoted as enzyme activity.
In practice, conventional values are used to characterize the activity of the enzyme: 1 international unit of activity (IU) corresponds to the amount of enzyme that catalyzes the conversion of 1 µmol of substrate in 1 minute under optimal conditions (temperature 37°C, optimal pH value of the solution) for the enzymatic reaction
reactions. These activity units are used in medical and pharmaceutical practice to assess enzyme activity:
To estimate the number of enzyme molecules among other proteins of a given tissue, determine the specific activity (Sp.A.) of the enzyme, numerically equal to the amount of converted substrate (in µmol) per unit time of one milligram (mg) of protein (enzyme isolated from the tissue):
The degree of purification of the enzyme is judged by the specific activity: the fewer foreign proteins, the higher the specific activity.
2. The kinetics of enzymatic reactions is studied under optimal conditions for the enzymatic reaction. Optimal conditions are individual for each enzyme and are determined primarily by the temperature at which the reaction is carried out and the pH value of the solution.
Temperature increase up to certain limits, it influences the rate of an enzymatic reaction in the same way that temperature influences any chemical reaction: with increasing temperature, the rate of the enzymatic reaction increases. However, the rate of an enzymatic chemical reaction has its own temperature optimum, the excess of which is accompanied by a decrease in enzymatic activity, which is associated with thermal denaturation of the protein molecule (Fig. 2.15). For most human enzymes, the optimal temperature is 37-38 °C.
 Rice. 2.15. Dependence of the enzymatic reaction rate (V) on temperature
Rice. 2.15. Dependence of the enzymatic reaction rate (V) on temperature
Enzyme activity depends on pH solution in which an enzymatic reaction occurs. The effect of pH on enzyme activity is due to changes in the ionization of functional groups of amino acid residues of a given protein and substrate, which ensure optimal formation of the enzyme-substrate complex. For each enzyme there is a pH value at which its maximum activity is observed (Fig. 2.16).
 Rice. 2.16. Dependence of the enzymatic reaction rate (V) on the pH of the medium
Rice. 2.16. Dependence of the enzymatic reaction rate (V) on the pH of the medium
3. The kinetic characteristics of an enzymatic reaction depend on the concentration of the reactants. If the concentration of the enzyme is left constant, changing only the amount of substrate, then the graph of the rate of the enzymatic reaction is described by a hyperbola (Fig. 2.17). As the amount of substrate increases, the initial reaction rate increases. When the enzyme becomes completely saturated with substrate, i.e. the maximum possible formation of enzyme-substrate complexes occurs at a given enzyme concentration, and the highest rate of product formation is observed. A further increase in the substrate concentration does not lead to an increase in the amount of product formed, i.e. the reaction rate does not increase. This state corresponds to the maximum reaction speed Vmax
The V max value characterizes the catalytic activity of the enzyme and determines the maximum possibility of product formation at a given enzyme concentration and under conditions of excess substrate; V max is a constant value for a given enzyme concentration.
 Rice. 2.17. Dependence of reaction rate (V) on substrate concentration S:
Rice. 2.17. Dependence of reaction rate (V) on substrate concentration S:
V max is the maximum reaction rate at a given enzyme concentration under optimal reaction conditions; K m - Michaelis constant
4. The main kinetic characteristic of enzyme efficiency is Michaelis constant - K m. The Michaelis constant is numerically equal to the substrate concentration at which half the maximum speed is achieved. K m characterizes the affinity of a given enzyme for a given substrate and is a constant value. The lower Km, the greater the affinity of the enzyme for a given substrate, the higher the initial reaction rate, and vice versa, the greater Km, the lower the affinity of the enzyme for the substrate and the lower the initial reaction rate.
1. Copy the table into your notebook. 2.2. Use your textbook and additional literature to fill out the table. Draw a conclusion about the need for a varied diet for human health.
2. Copy the table into your notebook. 2.3 and fill it out. Using your textbook, write down one reaction involving each coenzyme.
3. Transfer the enzyme activity graph to your notebook (Fig. 2.18). Define and indicate V max of these reactions. Specify K in the first and second
case. What is the biochemical meaning of the constant K?
Table 2.2. Characteristics of the main water-soluble vitamins that are precursors of coenzymes
 Table 2.3. Basic coenzymes
Table 2.3. Basic coenzymes

 Rice. 2.18. Dependence of the rate of enzymatic reactions on substrate concentration
Rice. 2.18. Dependence of the rate of enzymatic reactions on substrate concentration
SELF-CONTROL TASKS
1. Choose the correct answers. Enzymes:
A. Are proteins
B. Reduce the rate of enzymatic reactions
B. They have specificity of action D. They are simple proteins E. They are capable of regulation
2. Choose the correct answers. Michaelis constant (Km):
A. Is a characteristic of the substrate specificity of the enzyme B. Numerically equal to the substrate concentration at which half of Vmax is observed
B. Characterizes the affinity of the enzyme for the substrate
D. Characterizes the saturation of the active center of the enzyme with substrate D. Is a kinetic characteristic of the enzyme
3. Choose the correct answers. Coenzyme PF functions with enzymes of the following classes:
A. Oxidoreductase B. Transferase
B. Hydrolase G. Liaz D. Isomerases
4. Match. Type of reaction in which the coenzyme is involved:
A. Carboxylation B. Oxidation-reduction
B. Transamination D. Acylation E. Acetylation
Coenzyme:
2. Pyridoxal phosphate
5. Match. The enzyme catalyzes:
A. Only irreversible reactions
B. Same type reactions with a small number (group) of structurally similar substrates
B. Conversion of only one of the existing stereoisomers of the substrate
D. Reactions in the presence of coenzymes E. Conversion of only one substrate Substrate specificity:
1. Absolute
2. Group
3. Stereospecificity
6. Complete the "chain" task:
A) redox reactions are catalyzed by enzymes of the class
A. Transferases
B. Oxidoreductases
b) enzymes belonging to a subclass of this class carry out reactions
abstraction of hydrogen atoms from the substrate:
A. Oxidases
B. Hydroxylases
B. Dehydrogenases
V) The coenzyme for these enzymes is:
B. Coenzyme A
G) coenzyme is based on vitamin:
A. Nicotinic acid B. Biotin
B. Vitamin B 2
d) A deficiency of this vitamin leads to the following diseases:
B. Pellagra
B. Macrocytic anemia
7. Match. Enzyme class:
A. Oxidoreductase B. Hydrolase
B. Ligaza G. Liase
D. Transferase
Enzyme:
1. Succinate dehydrogenase
2. Pyruvate carboxylase.
3. DNase.
8. Complete the sentences with the missing words:
activity. A coenzyme linked to an apoenzyme by strong covalent bonds is called ..................
4. 1-A; 2-B; 3-B
5. 1-D; 2-B; 3-B
6. a) B; b) B; c) B; d) A; e) B
7. 1-A; 2-B; 3-B
8. Holoenzyme, apoenzyme, coenzyme, prosthetic group
BASIC TERMS AND CONCEPTS
1. Enzymology
2. Enzyme catalysis
3. Enzyme-substrate complex
4. Kinetics of enzymatic catalysis
5. Substrate
6. Enzyme active site
7. Maximum reaction speed - V max
8. Michaelis constant - K m
9. Enzyme activity units
10. Enzyme classes
11. Enzyme specificity
12. Enzyme cofactors
13. Specific activity of the enzyme
14. Apoenzyme
15. Holoenzyme
Solve problems
1. Currently, in biochemical laboratories, automatic biochemical analyzers are used to determine the activity of enzymes in human biological fluids. Help the laboratory technician understand the reagents to be used to determine lactate dehydrogenase (LDH) activity and calculate LDH activity in two patients. For this:
a) write the reaction catalyzed by LDH;
b) indicate the substrate, coenzyme, vitamin precursor, source of the enzyme;
c) list the reaction conditions (temperature, time);
d) explain by what parameter the rate of an enzymatic reaction can be assessed;
f) calculate LDH activity in the blood of patients in units of IU/l. Draw a conclusion: which patient is more active?
Table 2.4. Data for determining LDH activity
2. Humans are homeothermic (temperature is maintained at a constant level) living organisms. In medicine, extreme temperatures are used in some cases for treatment. In particular, hypothermic conditions are used for prolonged operations, especially on the brain and heart) hyperthermic conditions are used for the purpose of tissue coagulation. Explain the validity of these approaches from the point of view of an enzymologist. To answer:
a) indicate what temperature is optimal for most human enzymes;
b) draw a graph of the dependence of the rate of enzymatic reactions on temperature;
c) explain the need for long-term surgical interventions under hypothermic conditions;
d) describe what the method of thermal tissue coagulation is based on;
e) indicate the consequences of exposure to critical temperatures on humans.
3. A 35-year-old patient came to the clinic with complaints of inflammatory processes in the oral mucosa, muscle fatigue, and conjunctivitis. The patient ate a monotonous diet for a long time, excluding foods such as liver, rye, milk, and yeast from her diet. The doctor diagnosed hypovitaminosis B2. Explain the reasons for the observed symptoms. For this:
a) name the coenzymes formed from vitamin B2;
b) indicate in which reactions these coenzymes are involved;
c) write the working parts of the formula for the oxidized and reduced forms of coenzymes;
d) give examples of reactions involving these coenzymes (use textbook materials).
4. The enzyme acid phosphatase hydrolyzes phosphoric acid esters. This enzyme is formed in the cells of the liver, spleen, and prostate; it is contained by red blood cells, platelets, macrophages and osteoclasts. This enzyme is also contained in the acrosome of spermatozoa and, during fertilization, breaks down the phospholipids of the oocyte plasmalemma. The greatest enzymatic activity of acid phosphatase occurs at acidic pH values (4.7-6.0). Draw a graph of the reaction rate versus pH and explain why acid phosphatase activity changes with pH changes. Provide a diagram of the reaction. Determine the enzyme class and its specificity.
5. When studying the reaction rate of dipeptide conversion under the action of small intestinal peptidase, the following results were obtained: the maximum enzyme activity is 40 µmol/min/mg, Km 0.01. At what concentration of substrate is the reaction rate equal to 10 µmol/min/mg? Using task data:
a) write a reaction scheme, determine the class of enzyme and the bond that it destroys in the substrate;
b) draw a graph of the reaction rate depending on the substrate concentration and answer the question of the problem;
c) give the definition of Ksh, indicate the relationship between the value of Ksh and the affinity of the enzyme for the substrate.
6. The student determined the specific activity of the enzyme lysozyme isolated from chicken egg white. Lysozyme hydrolyzes glycoproteins of the bacterial cell wall. The student incubated a reaction mixture containing a substrate, an enzyme, and a buffer providing an optimal pH value of 5.2 and found that under the influence of 1 mg of lysozyme, only 12 µmol of product was formed in 15 minutes. Having made the calculation and finding out the reason
low specific activity of the enzyme, he remembered that he did not turn on the thermostat and therefore incubated the samples at room temperature, and the enzyme t was 37°C. Repeating the experiment under optimal conditions, he found that in 15 minutes, 45 µmol of product was formed by the action of 1 mg of lysozyme. Calculate the specific activity of the enzyme in both cases and explain the mechanism of the effect of temperature on the rate of the enzymatic reaction.
7. The activity of many enzymes in the cell is regulated by other enzymes - protein kinase and phosphoprotein phosphatase. Indicate the features of these reactions; write the reactions catalyzed by these enzymes, indicate which class of enzymes they belong to. Note substrate specificity.
Modular unit 2 REGULATION OF ENZYME ACTIVITY. MEDICAL ASPECTS OF ENZYMOLOGY
Learning objectives Be able to:
1. Interpret the results of the influence of inhibitors - drugs, poisons - on the enzymatic reactions of the body.
2. Explain the importance of regulation of enzyme activity in influencing the speed of a metabolic pathway.
3. Explain the basics of using enzymes as medicines.
4. Apply knowledge about the properties of enzymes and the enzyme composition of organs under normal conditions and in various metabolic disorders.
5. Interpret the results of determining enzyme activity in the diagnosis of diseases.
Know:
1. Classification of enzyme inhibitors according to their mechanism of action.
2. Examples of drugs - enzyme inhibitors.
3. Basic mechanisms for regulating enzyme activity in the body.
4. Principles of regulation of metabolic pathways and the role of enzymes in the regulation of metabolism.
5. Basics of the use of enzymes for the diagnosis and treatment of diseases.
TOPIC 2.7. ENZYME ACTIVITY INHIBITORS
1. Under the term "inhibition enzyme activity" refers to a specific decrease in catalytic activity caused by certain chemicals - inhibitors.
Inhibitors are of great interest for elucidating the mechanisms of enzymatic catalysis and helping to establish the role of individual enzymatic reactions in the metabolic pathways of the body. The action of many drugs and poisons is based on the principle of inhibition of enzymatic activity.
2. Inhibitors are able to bind to enzymes with varying degrees of strength. Based on this, they distinguish reversible And irreversible inhibition. Reversible inhibitors bind to the enzyme with weak non-covalent bonds and, under certain conditions, are easily separated from the enzyme:
E+I↔ EI.
Irreversible inhibition observed in the case of the formation of covalent stable bonds between the inhibitor molecule and the enzyme:
E+I→ E-I.
3. According to the mechanism of action, reversible inhibitors are divided into competitive And non-competitive.
Competitive inhibition causes a reversible decrease in the rate of the enzymatic reaction as a result of binding of the inhibitor to the active site of the enzyme, which prevents the formation of the enzyme-substrate complex. This type of inhibition occurs when the inhibitor is structural analogue of the substrate; As a result, competition between substrate and inhibitor molecules for binding to the active center of the enzyme occurs. In this case, either the substrate or the inhibitor interacts with the enzyme, forming enzyme-substrate (ES) or enzyme-inhibitor (EI) complexes. When an enzyme-inhibitor (EI) complex is formed, no reaction product is formed (Fig. 2.19).
 Rice. 2.19. Scheme of competitive inhibition of enzyme activity
Rice. 2.19. Scheme of competitive inhibition of enzyme activity
For the competitive type of inhibition, the following equations are valid:
E+S↔ES →E+P; E+I↔ E.I.
A distinctive feature of competitive inhibition is the possibility of its weakening with increasing substrate concentration, since a reversible inhibitor does not change the structure of the enzyme. Therefore, at high substrate concentrations, the reaction rate does not differ from that in the absence of an inhibitor, i.e. a competitive inhibitor does not change Vmax, but increases Km.
A classic example of competitive inhibition is the inhibition of the succinate dehydrogenase reaction by malonic acid (Fig. 2.20). Malonate is a structural analogue of succinate (presence of two carboxyl groups) and can also interact with the active site of succinate dehydrogenase. However, the transfer of two hydrogen atoms to the prosthetic group FAD from malonic acid is not possible and, therefore, the reaction rate is reduced.
 Rice. 2.20. An example of competitive inhibition of succinate dehydrogenase by malonic acid:
Rice. 2.20. An example of competitive inhibition of succinate dehydrogenase by malonic acid:
A - succinate binds to the active center of the enzyme succinate dehydrogenase due to ionic bonds; B - during the enzymatic reaction, two hydrogen atoms are removed from succinate and added to the coenzyme FAD. As a result, fumarate is formed, which is removed from the active site of succinate dehydrogenase; B - malonate is a structural analogue of succinate; it also binds to the active site of succinate dehydrogenase, but the chemical reaction does not occur
4. Many drugs exert their therapeutic effect through the mechanism of competitive inhibition. For example, the hydrolysis reaction of acetylcholine to choline and acetic acid is catalyzed by the enzyme acetylcholinesterase (AChE) (Fig. 2.21) and can be inhibited in the presence of competitive inhibitors of this enzyme (e.g. proserin, endrophonium etc.) (Fig. 2.22). When such inhibitors are added, the activity of acetylcholinesterase decreases, the concentration of acetylcholine (substrate) increases, which is accompanied by an increase in the conduction of nerve impulses. Competitive acetylcholine esterase inhibitors are used in the treatment of muscular dystrophies, as well as for the treatment of movement disorders after injuries, paralysis, and poliomyelitis.
Rice. 2.21. The reaction of acetylcholine hydrolysis under the influence of AChE
 Rice. 2.22. Binding of competitive inhibitors in the active site of AChE
Rice. 2.22. Binding of competitive inhibitors in the active site of AChE
A - addition of a substrate (acetylcholine) to the active center of the enzyme.
The arrow indicates the site of acetylcholine hydrolysis; B - addition of the competitive inhibitor proserin to the active center of the enzyme. There is no reaction; B - attachment of the competitive inhibitor endrophonium to the active center of the enzyme. The attachment of inhibitors to the active site of AChE prevents the binding of acetylcholine
Another example of drugs whose mechanism of action is based on competitive inhibition of the enzyme is the use of peptide inhibitors of the proteolytic enzyme trypsin for diseases of the pancreas (acute pancreatitis, necrosis), such as aprotinin, trasylol, contrical. These drugs inhibit trypsin, which is released into surrounding tissues and blood, and thereby prevent unwanted autolytic events in pancreatic diseases.
5. In some cases, competitive inhibitors, interacting with the active center of the enzyme, can be used as pseudosubstrates(antimetabolites), which leads to the synthesis of a product with an incorrect structure. The resulting substances do not have the “desired” structure and therefore lack functional activity. These drugs include sulfonamide drugs.
6. Non-competitive Reversible is the inhibition of an enzymatic reaction in which the inhibitor interacts with the enzyme at a site other than the active site. Noncompetitive inhibitors are not structural analogues of the substrate; the addition of a noncompetitive inhibitor to the enzyme changes the conformation of the active center and reduces the rate of the enzymatic reaction, i.e. reduces enzymatic activity. An example of a non-competitive inhibitor may be the action of heavy metal ions, which interact with the functional groups of the enzyme molecule, interfering with catalysis.
7. Irreversible inhibitors reduce enzymatic activity as a result of the formation of covalent bonds with the enzyme molecule. Most often, the active center of the enzyme undergoes modification. As a result, the enzyme cannot perform its catalytic function.
The use of irreversible inhibitors is of greater interest for elucidating the mechanism of action of enzymes. Important information about the structure of the active center of an enzyme is provided by compounds that block certain groups of the active center. Such inhibitors are called specific. Specific inhibitors include diisopropyl fluorophosphate (DFP). DPP forms a covalent bond with the OH group of serine, which is contained in the active center of the enzyme and is directly involved in catalysis, therefore DPP is classified as a specific irreversible inhibitor of “serine” enzymes (Fig. 2.23). DPP is used to study the structure of the active center of enzymes in enzymology.
Unlike specific inhibitors nonspecific inhibitors form covalent bonds with certain enzyme groups located not only in the active center, but also in any part of the enzyme molecule. For example, iodine acetate (Fig. 2.24) interacts with any SH groups of the protein. This interaction changes the conformation of the enzyme molecule, and, accordingly, the conformation of the active center and reduces catalytic activity.
 Rice. 2.23. Specific inhibition of chymotrypsin activity using DPP
Rice. 2.23. Specific inhibition of chymotrypsin activity using DPP
 Rice. 2.24. Nonspecific inhibition of enzyme activity by iodine acetate.
Rice. 2.24. Nonspecific inhibition of enzyme activity by iodine acetate.
Nonspecific inhibition occurs due to covalent modification of cysteine SH groups by iodine acetate molecules
8. An example of a drug whose action is associated with irreversible enzyme inhibition is the widely used aspirin. The action of this anti-inflammatory non-steroidal drug is based on inhibition of the enzyme cyclooxygenase, which catalyzes the formation of prostaglandins from arachidonic acid. As a result, the acetyl residue of aspirin is added to the free terminal OH group of serine of one of the subunits of cyclooxygenase (Fig. 2.25). This blocks the formation of prostaglandins (see module 8), which have a wide range of biological functions, including mediators of inflammation. Therefore, aspirin is classified as an anti-inflammatory drug. Inhibited enzyme molecules are destroyed, prostaglandin synthesis is restored only after the synthesis of new enzyme molecules.
 Rice. 2.25. The mechanism of cyclooxygenase inactivation using an irreversible inhibitor - aspirin
Rice. 2.25. The mechanism of cyclooxygenase inactivation using an irreversible inhibitor - aspirin
TOPIC 2.8. REGULATION OF ENZYME ACTIVITY
1. All chemical reactions in a cell occur with the participation of enzymes. Therefore, in order to influence the rate of the metabolic pathway (the sequential transformation of one substance into another), it is enough to regulate the number of enzyme molecules or their activity. Usually in metabolic pathways there are key enzymes due to which the speed of the entire path is regulated. These enzymes (one or more in a metabolic pathway) are called regulatory enzymes. Regulation of the rate of enzymatic reactions is carried out at three independent levels: by changing the number of enzyme molecules, the availability of substrate and coenzyme molecules, and changing the catalytic activity of the enzyme molecule (Table 2.6).
Table 2.5. Methods for regulating the rate of enzymatic reactions
Method of regulation | Characteristic |
Change in the number of enzyme molecules | The number of enzyme molecules in a cell is determined by the ratio of two processes: synthesis and decay. The most studied mechanism of regulation of enzyme synthesis is at the level of transcription (mRNA synthesis), which is regulated by certain metabolites, hormones and a number of biologically active molecules. |
Availability of substrate and coenzyme molecules | An important parameter that controls the course of an enzymatic reaction is the presence of substrate and coenzyme. The higher the concentration of the starting substrate, the higher the reaction rate |
Change in the catalytic activity of an enzyme molecule | The main ways to regulate enzyme activity are: Allosteric regulation; Regulation by protein-protein interactions; Regulation by phosphorylation-dephosphorylation of the enzyme molecule; Regulation by partial (limited) proteolysis |
Let's consider ways to regulate the rate of enzymatic reactions by changing the catalytic activity of the enzyme molecule.
2. Allosteric regulation. Allosteric enzymes called enzymes, activity which can be adjusted by using effector substances. The effectors involved in allosteric regulation are cellular metabolites that are often participants in the very pathway they regulate.
The effector that causes reduction (inhibition) enzyme activity is called inhibitor. The effector that causes increase (activation) enzyme activity is called activator.
Allosteric enzymes have certain structural features:
Usually are oligomeric proteins, consisting of several protomers;
Have allosteric center, spatially distant from the catalytic active site;
Effectors attach to the enzyme non-covalently at allosteric (regulatory) centers.
Allosteric centers, like catalytic ones, can exhibit different specificity with respect to ligands: it can be absolute or group. Some enzymes have several allosteric centers, some of which are specific to activators, others to inhibitors.
The protomer on which the allosteric center is located is called regulatory protomer Unlike catalytic protomer, containing an active center in which a chemical reaction takes place.
Allosteric enzymes have the property cooperativeness: the interaction of an allosteric effector with an allosteric center causes a cooperative change in the conformation of all subunits, leading to a change in the conformation of the active center and a change in the affinity of the enzyme for the substrate, which reduces or increases the catalytic activity of the enzyme. If an inhibitor is attached to the allosteric center, then as a result of cooperative conformational changes, a change in the conformation of the active center occurs, which causes a decrease in the affinity of the enzyme for the substrate and, accordingly, a decrease in the rate of the enzymatic reaction. Conversely, if an activator is attached to the allosteric center, then the affinity of the enzyme for the substrate increases, which causes an increase in the reaction rate. The sequence of events under the action of allosteric effectors is presented in Fig. 2.26.
Regulation of allosteric enzymes reversible: detachment of the effector from the regulatory subunit restores the original catalytic activity of the enzyme.
Allosteric enzymes catalyze key reactions of this metabolic pathway.
Allosteric enzymes play an important role in various metabolic pathways, as they respond extremely quickly to the slightest changes in the internal composition of the cell. The speed of metabolic processes depends on the concentration of substances, both used and formed in a given chain of reactions. Precursors can be activators of allosteric enzymes in the metabolic pathway. At the same time, when the end product of any metabolic pathway accumulates, it can act as an allosteric inhibitor of the enzyme. This method of regulation is common in the body and is called “negative feedback”:
 Rice. 2.26. Scheme of the structure and functioning of an allosteric enzyme:
Rice. 2.26. Scheme of the structure and functioning of an allosteric enzyme:
A - the action of a negative effector (inhibitor). The inhibitor (I) attaches to the allosteric center, which causes cooperative conformational changes in the enzyme molecule, including in the active center of the enzyme. The affinity of the enzyme for the substrate decreases, and as a result, the rate of the enzymatic reaction decreases; B - action of a positive effector (activator). The activator (A) binds to the allosteric center, which causes cooperative conformational changes. The affinity of the enzyme for the substrate increases and the rate of the enzymatic reaction increases. The reversible effect of both inhibitor and activator on enzyme activity has been demonstrated
Let's consider the allosteric regulation of the process of glucose catabolism, which ends with the formation of an ATP molecule (Fig. 2.27). In the event that ATP molecules in the cell are not consumed, it is an inhibitor of allosteric enzymes of this metabolic pathway: phosphofructokinase and pyruvate kinase. At the same time, the intermediate metabolite of glucose catabolism, fructose-1,6-bisphosphate, is an allosteric activator of the pyruvate kinase enzyme. Inhibition by the end product of the metabolic pathway and activation by the initial metabolites allows
 Rice. 2.27. Allosteric regulation of the process of glucose catabolism.
Rice. 2.27. Allosteric regulation of the process of glucose catabolism.
The ATP molecule is an allosteric inhibitor of metabolic pathway enzymes - phosphofructokinase and pyruvate kinase. The fructose-1,6-bisphosphate molecule is an allosteric activator of the enzyme pyruvate kinase
regulate the speed of the metabolic pathway. Allosteric enzymes catalyze, as a rule, the initial reactions of a metabolic pathway, irreversible reactions, rate-limiting reactions (the slowest) or reactions at the branch point of a metabolic pathway.
3. Regulation by protein-protein interactions. Some enzymes change their activity as a result of protein-protein interactions. At least two mechanisms for changing enzyme activity in this way can be distinguished: activation of enzymes as a result of the addition of activator proteins (activation of the enzyme adenylate cyclase by the α-subunit of the G protein, see module 4) and changes in catalytic activity as a result of association and dissociation of protomers.
As an example of the regulation of the catalytic activity of enzymes by association or dissociation of protomers, we can consider the regulation of the enzyme protein kinase A.
Protein kinase A(cAMP-dependent) consists of four subunits of two types: two regulatory (R) and two catalytic (C). This tetramer does not have catalytic activity. Regulatory subunits have binding sites for cyclic 3",5"-AMP (cAMP) (two for each subunit). The attachment of four cAMP molecules to two regulatory subunits leads to a change in the conformation of the regulatory protomers and to the dissociation of the tetrameric complex; this releases two active catalytic subunits (Fig. 2.28). Active protein kinase A catalyzes the transfer of a phosphoric acid residue from ATP to specific OH groups of amino acid residues of proteins (i.e., it causes phosphorylation of proteins).
 Rice. 2.28. Regulation of protein kinase A (PKA) activity by protein-protein interactions.
Rice. 2.28. Regulation of protein kinase A (PKA) activity by protein-protein interactions.
PKA is activated by four cAMP molecules, which bind to two regulatory subunits, which leads to a change in the conformation of the regulatory protomers and dissociation of the tetrameric complex. This releases two active catalytic subunits that can cause protein phosphorylation
The cleavage of cAMP molecules from the regulatory subunits leads to the association of the regulatory and catalytic subunits of proten kinase A with the formation of an inactive complex.
4. Regulation of the catalytic activity of enzymes by phosphorylation-dephosphorylation. In biological systems, a mechanism for regulating the activity of enzymes using their covalent modification is often found. A quick and widespread method of chemical modification of enzymes is their phosphorylation-dephosphorylation.
The OH groups of the enzyme undergo phosphorylation, which is carried out by enzymes protein kinases(phosphorylation) and phosphoprotein phosphatases(dephosphorylation). The addition of a phosphoric acid residue leads to a change in the conformation of the active center and its catalytic activity. In this case, the result can be twofold: some enzymes are activated during phosphorylation, while others, on the contrary, become less active (Fig. 2.29). The activity of protein kinases and phosphoprotein phosphatases is regulated by hormones, which allows the activity of key enzymes in metabolic pathways to rapidly vary depending on environmental conditions.
 Rice. 2.29. Scheme of regulation of enzyme activity by phosphorylation-dephosphorylation.
Rice. 2.29. Scheme of regulation of enzyme activity by phosphorylation-dephosphorylation.
Phosphorylation of enzymes occurs with the help of the enzyme protein kinase. The donor of the phosphoric acid residue is the ATP molecule. Phosphorylation of an enzyme changes its conformation and the conformation of the active site, which changes the affinity of the enzyme for the substrate. In this case, some enzymes are activated during phosphorylation, while others are inhibited. The reverse process - dephosphorylation - is caused by the enzymes phosphoprotein phosphatases, which cleave off the phosphoric acid residue from the enzyme and return the enzyme to its original state
5. Regulation of the catalytic activity of enzymes by partial (limited) proteolysis. Some enzymes that function outside cells (in the gastrointestinal tract or blood plasma) are synthesized as inactive precursors and are activated only as a result of hydrolysis of one or more specific peptide bonds, which leads to the elimination of part of the molecule. In the remaining part of the protein molecule, a conformational rearrangement occurs and the active center of the enzyme is formed (Fig. 2.30). Partial proteolysis is an example of regulation when the activity of an enzyme is changed
 Rice. 2.30. Activation of pepsin by partial proteolysis.
Rice. 2.30. Activation of pepsin by partial proteolysis.
As a result of hydrolysis of one or more peptide bonds of pepsinogen (an inactive molecule), part of the molecule is split off and the active center of the pepsin enzyme is formed
irreversible. Such enzymes usually function for a short time, determined by the lifetime of the protein molecule. Partial proteolysis underlies the activation of digestive proteolytic enzymes (pepsin, trypsin, chymotrypsin, elastase), peptide hormones (insulin), proteins of the blood coagulation system and a number of other proteins.
TOPIC 2.9. APPLICATION OF ENZYMES IN MEDICINE
1. Enzymes are widely used in medical practice as diagnostic (enzymodiagnostics) and therapeutic (enzyme therapy) funds. Enzymes are also used as specific reagents
to determine a number of metabolites. For example, the enzyme glucose oxidase is used for the quantitative determination of glucose in urine and blood; the enzyme urease is used to assess the urea content in biological fluids; using various dehydrogenases, the presence of appropriate substrates is detected, for example, pyruvate, lactate, ethyl alcohol, etc.
2. Enzymodiagnostics consists of diagnosing a disease (or syndrome) based on determining the activity of enzymes in human biological fluids.
The principles of enzyme diagnostics are based on the following principles:
Normally, blood serum contains enzymes that perform specialized functions, for example, those involved in the blood coagulation system. Cellular enzymes practically do not penetrate from intact cells into the blood. In minimal quantities, some cell enzymes can be detected in the blood;
At damage cell membranes (inflammation, necrosis) in the blood or other biological fluids (for example, urine), the number of intracellular enzymes of damaged cells increases, the activity of which can be recorded by special biochemical tests;
For enzymatic diagnostics, enzymes that have a predominant or absolute localization in certain organs are used. (organ specificity);
The amount of enzyme released should be proportional to the degree of tissue damage and sufficient to determine its activity;
The activity of enzymes in biological fluids detected when cells are damaged differs from normal values and is stable for quite a long time (days);
The appearance in the blood plasma of enzymes that have only cytosolic localization indicates an inflammatory process; if mitochondrial or nuclear enzymes are detected, we can talk about deeper cell damage, such as necrosis.
Enzymes that catalyze the same chemical reaction but with different primary protein structures are called isozymes. They differ from each other in kinetic parameters, activation conditions, and characteristics of the connection between the apoenzyme and the coenzyme. The nature of the appearance of isoenzymes is varied, but most often due to differences in the structure of the genes encoding these isoenzymes or their subunits. Methods for determining isoenzymes are based on differences in physicochemical properties. Isoenzymes are often organ-specific, since each tissue contains predominantly one type of isoenzymes. Consequently, when an organ is damaged, the corresponding form of the isoenzyme appears in the blood. The detection of certain isoenzyme forms of enzymes allows their use for diagnosing diseases.
For example, an enzyme lactate dehydrogenase (LDH) catalyzes the reversible oxidation reaction of lactate (lactic acid) to pyruvate (pyruvic acid) (Fig. 2.31). Lactate dehydrogenase is an oligomeric protein with a mol. weighing 134,000, consisting of four subunits of two types - M (from the English muscle - muscle) and H (from the English heart - heart). The combination of these subunits underlies the formation of five isoforms of lactate dehydrogenase (Fig. 2.32, A). LDH 1 and LDH 2 are most active in the heart muscle and kidneys, LDH 4 and LDH 5 - in skeletal muscles and liver. Other tissues contain other variants of this enzyme. LDH isoforms differ from each other in electrophoretic mobility, which makes it possible to establish the tissue identity of LDH isoforms (Fig. 2.32, B). To diagnose diseases of the heart, liver and muscles, it is necessary to study LDH isoforms in blood plasma using electrophoresis. In Fig. 2.32, B shows electropherograms
 Rice. 2.31. Reaction catalyzed by lactate dehydrogenase (LDH)
Rice. 2.31. Reaction catalyzed by lactate dehydrogenase (LDH)
 Rice. 2.32. Lactate dehydrogenase isoforms:
Rice. 2.32. Lactate dehydrogenase isoforms:
A - structure of various LDH isoforms; B - distributions on the electropherogram and relative amounts of LDH isoforms in various organs; B - content of LDH isoforms in blood plasma in normal conditions and in pathology (electropherograms - on the left and photometric scanning - on the right)
blood plasma of a healthy person, a patient with myocardial infarction and a patient with hepatitis. Detection of tissue-specific LDH isoforms in blood plasma is widely used as a diagnostic test.
Another example is creatine kinase. Creatine kinase (CK) which catalyzes the reaction of creatine phosphate formation (Fig. 2.33). The KK molecule is a dimer consisting of two types of subunits M (from the English muscle - muscle) and B (from the English brain - brain). These subunits form three isoenzymes: BB, MB, MM. The BB isoenzyme is found primarily in the brain, MM in skeletal muscles, and MV in cardiac muscle. KK isoforms have different electrophoretic mobilities (Fig. 2.34). Determination of CK activity in blood plasma is important in the diagnosis of myocardial infarction (there is an increase in the level of the MB isoform). The amount of the MM isoform may increase during trauma and damage to skeletal muscles. The BB isoform cannot penetrate the blood-brain barrier, therefore it is practically undetectable in the blood even during strokes and has no diagnostic value.
 Rice. 2.33. Reaction catalyzed by the enzyme creatine kinase (CK)
Rice. 2.33. Reaction catalyzed by the enzyme creatine kinase (CK)
 Rice. 2.34. Structure and electrophoretic mobility of various creatine kinase isoforms
Rice. 2.34. Structure and electrophoretic mobility of various creatine kinase isoforms
Enzymodiagnostics used to establish a diagnosis for diseases of various organs. The set of analyzes depends on the capabilities of a particular biochemical laboratory and is constantly being improved. The most common enzyme diagnostic tests are:
For heart diseases (myocardial infarction) - lactate dehydrogenase, creatine kinase, aspartate aminotransferase, alanine aminotransferase. One of the first proteins to appear in the blood during myocardial infarction is troponin;
For liver diseases - alanine aminotransferase, aspartate aminotransferase, acetylcholinesterase, gamma-glutamyl transpeptidase. For diseases of the pancreas - pancreatic amylase, lipase;
For prostate diseases - acid phosphatase.
3. Use of enzymes as medicines are actively developing in the following directions:
Replacement therapy - the use of enzymes in case of their deficiency;
Elements of complex therapy - the use of enzymes in combination with other therapy.
Enzyme replacement therapy is effective for gastrointestinal diseases associated with insufficient secretion of digestive juices. For example, pepsin is used for gastritis with reduced secretory function. Deficiency of pancreatic enzymes can also be compensated to a large extent by ingesting drugs containing the main pancreatic enzymes (festal, enzistal, mesimforte, etc.).
Enzymes are used as additional therapeutic agents for a number of diseases. Proteolytic enzymes (trypsin, chymotrypsin) are used locally to treat purulent wounds in order to break down the proteins of dead cells, to remove blood clots or viscous secretions in inflammatory diseases of the respiratory tract. Enzyme preparations ribonuclease and deoxyribonuclease are used as antiviral drugs in the treatment of adenoviral conjunctivitis and herpetic keratitis.
Enzyme preparations have become widely used in thrombosis and thromboembolism to destroy the blood clot. For this purpose, fibrinolysin, streptolyase, streptodecase, and urokinase preparations are used.
The enzyme hyaluronidase (lidase), which catalyzes the breakdown of hyaluronic acid, is used subcutaneously and intramuscularly to resolve adhesions and scars after burns and operations.
The enzyme asparaginase (destroys the amino acid Asn in the blood) is used for cancer of the blood, limiting the flow of the amino acid Asn into tumor cells. Leukemia cells are not capable of independently synthesizing this amino acid, so a decrease in its content in the blood impairs the growth of these cells.
TOPIC 2.10. ENZYMOPATHIES
The basis of many diseases is the disruption of the functioning of enzymes in the cell - the so-called enzymopathies. Enzymopathies are distinguished between primary (hereditary) and secondary (acquired). Acquired enzymopathies, like proteinopathies in general, appear to be observed in all diseases.
In primary enzymopathies, defective enzymes are inherited mainly in a recessive autosomal manner. In this case, the metabolic pathway containing the defective enzyme is disrupted (Fig. 2.35). The development of the disease in this case can occur according to one of the “scenarios”:
The formation of final products is disrupted, which causes a lack of certain substances (for example, with albinism, pigment is not produced in skin cells);
Precursor substrates accumulate, which have a toxic effect on the body (for example, with alkaptonuria, an intermediate metabolite accumulates - homogentesic acid, which is deposited in the joints, causing inflammatory processes in them).
 Rice. 2.35. Metabolic pathway with enzyme E 3 enzymopathy
Rice. 2.35. Metabolic pathway with enzyme E 3 enzymopathy
ASSIGNMENTS FOR EXTRACURRICULAR WORK
Solve problems
1. In adipose tissue cells, the switching of metabolic processes from anabolic to catabolic occurs depending on the rhythm of nutrition. Hormones that regulate the activity of key enzymes by phosphorylation-dephosphorylation play an important role in the regulation of this switch. Complete the scheme for regulating the activity of the key fat breakdown enzyme (Fig. 2.36), if it is known that this enzyme (TAG lipase) is active in the phosphorylated form and inactive in the dephosphorylated form. To answer the question:
a) copy the diagram into a notebook and indicate the names of enzymes that cause phosphorylation and dephosphorylation of proteins (write their names in the rectangles);
b) name the class of these enzymes;
c) write down additional substrates and products involved in these reactions (write their names in the squares);
d) draw a conclusion about the role of hormones in the regulation of cell metabolism.
 Rice. 2.36. Regulation of TAG lipase activity
Rice. 2.36. Regulation of TAG lipase activity
2. Asparaginase, which catalyzes the reaction of asparagine catabolism, has found application in the treatment of leukemia. The prerequisite for the anti-leukemic effect of asparaginase was the fact that a defective enzyme for asparagine synthesis, asparagine synthetase, was identified in leukemic cells. Justify the therapeutic effect of asparaginase. To answer:
a) write the reactions catalyzed by the enzymes asparagine synthetase (section 7) and asparaginase;
b) indicate the classes to which these enzymes belong;
c) draw a conclusion about the concentration of Asn in tumor cells when using asparaginase;
d) explain why the use of asparaginase reduces the growth rate of tumor tissue.
3. Transfer it to your notebook and fill out the table. 2.7 on the use of enzymes in medicine using the material from this manual, textbook.
4. Transfer it to your notebook and fill out the table. 2.8 on drugs - enzyme inhibitors, using the current section, textbook, additional literature.
Table 2.7. Medicines - enzyme inhibitors
SELF-CONTROL TASKS
1. Choose the correct answer.
Competitive inhibitors:
A. Form covalent bonds with the active center of the enzyme B. Interact with the allosteric center
B. Interact with the active site of the enzyme, forming weak bonds
D. Reduce K w D. Reduce V max
2. Choose the correct answer. Irreversible inhibitors:
A. They are structural analogs of the substrate B. They form covalent bonds with the enzyme
B. Form weak bonds with the enzyme
D. Interact with the regulatory center
D. Reduce their effect with increasing substrate concentration
3. Choose the correct answers. Allosteric enzymes are generally:
A. They are proteins with a tertiary structure
B. Consist of several protomers C. Irreversibly inhibited
D. They have active and allosteric centers located on different protomers
D. Regulated by metabolites of this process
4. Choose the correct answers.
When enzymes are regulated by partial proteolysis, the following occurs:
A. Shortening of the protein peptide chain
B. Changes in the secondary and tertiary structure of the enzyme
B. Irreversible activation
D. Irreversible inhibition
D. Formation of the active center
5. Choose the correct answer.
Regulation of enzyme activity through protein-protein interactions is accompanied by:
A. Irreversible inhibition
B. Attachment or detachment of regulatory protein subunits
B. Attachment of an effector molecule to the allosteric center D. Phosphorylation of the enzyme
D. Dephosphorylation of the enzyme
6. Choose the correct answers. Enzymodiagnostics is based on:
A. Release of enzymes into the blood during tissue damage B. Organ specificity
B. High enzyme stability
D. The predominance of certain isoenzymes in different tissues D. Low activity or complete absence of activity of diagnostically significant enzymes in the blood is normal
7. Match.
Used to diagnose diseases:
B. Prostate gland
B. Pancreas D. Kidney
D. Hearts Enzyme:
1. Creatine kinase
2. Amylase
3. Acid phosphatase
8. Complete the "chain" task:
a) one of the enzymes determined during the enzymatic diagnosis of myocardial infarction is:
A. Acid phosphatase B. Lactate dehydrogenase
B. Amylase
b) this enzyme belongs to the class of enzymes:
A. Hydrolase B. Ligase
B. Oxidoreductase
V) one of the coenzymes of this class of enzymes is:
A. Pyridoxal phosphate B. Biotin
G) The vitamin that is the precursor of this coenzyme is:
A. Nicotinic acid B. Pyridoxine
9. Complete the “chain” task:
A) After poisoning with organic fluorophosphates, a person experiences:
A. Pupil dilation
B. Increased contraction of smooth muscles
B. Relaxation of smooth muscles
b) The reason for this effect is due to:
A. Impaired functioning of Na+, E+-ATPase B. Increased amount of acetylcholine
B. Reducing the amount of acetylcholine
V) This is due to the fact that fluorophosphates:
A. They are a competitive inhibitor of acetylcholinesterase (AChE)
B. Form covalent bonds with AChE
B. Disturb the synthesis of acetylcholine
G) This method of inhibition is called:
A. Irreversible B. Reversible
B. Competitive
d) a similar method of inhibition is observed when using:
A. Trasylol B. Aspirin
B. Proserina
STANDARDS OF ANSWERS TO “SELF-CONTROL TASKS”
3. B, G, D
4. A, B, C, D
6. A, B, D, D
7. 1-D, 2-B, 3-B
8. a) B, b) C, c) C, d) A
9. a) B, b) B, c) B, d) A, e) B
BASIC TERMS AND CONCEPTS
1. Metabolic pathway
2. Enzyme inhibition
3. Enzyme activation
4. Reversible inhibition
5. Irreversible inhibition
6. Competitive inhibition
7. Allosteric regulation
8. Allosteric effectors
9. Key enzymes
10. Regulation by forsphorylation - dephosphorylation
11. Regulation by protein-protein interactions
12. Partial proteolysis
13. Isoenzymes
14. Enzymopathy
15. Enzymodiagnostics
TASKS FOR CLASSROOM WORK
Solve problems
1. In human cells, the metabolic pathway for the synthesis of purine nucleotides necessary for the synthesis of nucleic acids begins with a molecule of ribose-5-phosphate. During the synthesis process, at a certain stage, this process branches and ends with the formation of two purine nucleotides - AMP and GMP (Fig. 2.37). To form equimolar ratios of these nucleotides in the cell, there is a multi-stage regulation of several key enzymes using a negative feedback mechanism. Thus, with an excess of AMP formation, the formation of adenylosuccinate slows down, and with an excess of GMP, the formation of xanthosine monophosphate slows down. At the same time, if both of these nucleotides are not consumed, the formation of phosphoribosyl diphosphate slows down. Guess which enzymes of the metabolic pathway for the synthesis of purine nucleotides are regulatory. To answer:
a) give definitions: “metabolic pathway” and “key enzymes of the metabolic pathway”;
b) guess which of the enzymes shown in Fig. 2.37 are regulatory;
c) indicate the mechanism of regulation of these enzymes, their localization in the metabolic pathway and structural features;
d) name which compounds and for which enzymes are effectors;
e) justify the concept of regulation “by a negative feedback mechanism”.

Rice. 2.37. Scheme of the formation of purine nucleotides in a cell
2. In 1935, the German doctor G. Domagk discovered the antimicrobial effect of protonsil (red streptocide), synthesized as a dye. It was soon established that the active principle of red streptocide is the sulfonamide (streptocide) formed during its metabolism, which was the ancestor of a large group of sulfonamide drugs (Fig. 2.38).
 Rice. 2.38. Structure of folic acid and general formula of sulfonamides
Rice. 2.38. Structure of folic acid and general formula of sulfonamides
The bacteriostatic effect of sulfonamides is that they replace para-aminobenzoic acid (PABA) in the active center of the enzyme dihydropteorate synthase during the synthesis of folic acid by bacteria, which is necessary for the formation of nucleic acids; as a result, the growth and development of microorganisms is disrupted. Folic acid is not synthesized in the human body, but is supplied with food as a vitamin.
Explain the mechanism of the antibacterial action of sulfonamides; to do this, answer the questions:
a) what is this type of inhibition called (compare the structures of sulfonamides and PABA)? How do such inhibitors affect Kt and Vmax
c) why is a loading dose of sulfonamides usually immediately prescribed during treatment?
d) will sulfonamides affect the formation of nucleic acids in human cells? Explain your answer.
3. 2 patients suffering from depressive disorders consulted a psychiatrist. It is known that the cause of depression in humans in some cases is a lack of neurotransmitters in the synaptic cleft. Also in the brain there are enzymes of the monoamine oxidases (MAO) group, which destroy neurotransmitters released into the synaptic cleft. The first patient was prescribed pirlindole, which is a structural analogue of the mediator serotonin. The second is nialamide, which is capable of covalently binding to the active site of MAO. Explain the mechanisms of action of these drugs and indicate which patient is most likely to respond more quickly to the drug. To answer:
a) characterize the effect of these drugs on MAO, indicate the difference in
mechanisms of interaction with this enzyme;
b) give a scheme for MAO inhibition by pirlindole and nialamide;
c) based on the mechanism of inhibition of these drugs, explain
which one will have a longer lasting effect on the body and why.
4. Recently, there has been an increase in the use of methanol for the production of technical fluids used in vehicle care products, including windshield washer agents. The main danger of methyl alcohol, or methanol, is its use as a surrogate alcohol, which leads to death. Thus, according to the Scientific and Practical Toxicology Center of Roszdrav, the proportion of patients poisoned by methanol ranges from 0.1 to 0.5% of all hospitalized patients. Explain the cause of methanol toxicity and how to provide medical treatment if it is known that methanol inhibits the activity of the enzyme acetaldehyde dehydrogenase, which is involved in the catabolism of ethanol, which causes the accumulation of acetaldehyde. To answer the question:
a) write the oxidation reactions of ethanol, taking into account that oxidation occurs
goes in two stages with the formation of an intermediate compound - acetaldehyde; the final product is acetic acid; the coenzyme of both reactions is NAD+;
b) write the structural formula of methanol and indicate the mechanism of inhibition of enzyme activity;
c) suggest a method of treatment in cases of methanol poisoning.
5. In the old days, Italian ladies dropped belladonna juice into their eyes, which caused the pupils to dilate and the eyes to acquire a special shine. It is now known that a similar effect is caused by the alkaloid atropine, contained in many plants: belladonna, henbane, datura. Explain the mechanism of action of atropine. For this:
a) name the receptors that atropine inhibits (see module 1), indicate the types of receptors and the sequence of events when atropine gets into the eyes;
b) answer where atropine and drugs with similar effects are used in medicine;
c) indicate what measures can be taken in case of an overdose of atropine? Justify possible ways to increase the concentration of acetylcholine and explain the need for this action.
6. Use of large doses caffeine causes symptoms in people similar to the effects of adrenaline: increased heart rate; bronchial dilatation, excitement, changes in metabolism in tissues that deposit energy carriers. Explain the mechanism of action of caffeine, bearing in mind that it is a competitive inhibitor of the enzyme phosphodiesterase (PDE), responsible for the breakdown of cAMP:
To answer this question:
a) answer, the concentration of which substance will increase in the cell under the influence of caffeine;
b) explain the mechanism of the regulatory action of cAMP in the cell; schematically depict the structure of the enzyme, which is activated due to an increase in the concentration of cAMP in the cell;
c) name what processes in the cell will be activated as a result of caffeine use? Write a diagram of these reactions;
d) remember that a similar mechanism of action is observed in drugs that improve the rheological properties of blood (for example, trental), as well as drugs that are used to relax the bronchi and relieve bronchospasm (for example, theophylline).
7. Patient L. was admitted to the hospital with suspected myocardial infarction. According to the patient, 5 hours before the doctor’s arrival, he experienced shortness of breath. The doctor suspected myocardial infarction and hospitalized the patient. In the hospital, a biochemical blood test was performed over several days to confirm the diagnosis. The results of the analyzes are presented in table. 2.9. Do the data obtained confirm the doctor’s diagnosis? To answer:
Enzyme | Activity, IU/l | Multiplicity | Activity, IU/l | Multiplicity |
12 hours after vessel occlusion | 72 hours after vessel occlusion |
|||
24 hours after vessel occlusion | 96 hours after vessel occlusion |
|||
48 hours after vessel occlusion | 120 hours after vessel occlusion |
|||
Three types of mechanisms are involved in the regulation of metabolic pathways. The first of them, which responds most quickly to any change in the situation, is associated with the action of allosteric enzymes (Fig. 13-15), the catalytic activity of which can change under the influence of special substances that have a stimulating or inhibitory effect (they are called effectors or modulators; section 9.18 ).
As a rule, allosteric enzymes occupy a place at the beginning or near the beginning of a given multienzyme sequence and catalyze that stage that limits the rate of the entire process as a whole; Usually the role of such a stage is played by a practically irreversible reaction.
Rice. 13-15. Regulation of the catabolic pathway by feedback type, i.e. due to inhibition of the allosteric enzyme by the end product of this process. The letters J, K, L, etc. indicate intermediate products of this metabolic pathway, and the letters E1, E2, E3, etc., indicate enzymes that catalyze individual stages. The first step is catalyzed by an allosteric enzyme (ED) which is inhibited by the end product of this reaction sequence. Allosteric inhibition is indicated by a broken red arrow that connects the inhibitory metabolite to the reaction catalyzed by the allosteric enzyme. The regulated step (catalyzed by the EJ enzyme is usually a virtually irreversible reaction under cellular conditions.
In catabolic processes accompanied by the synthesis of ATP from ADP, this final product, ATP, often acts as an allosteric inhibitor of one of the early stages of catabolism. An allosteric inhibitor of one of the early stages of anabolism is often the final product of biosynthesis, for example, some amino acid (Section 9.18). The activity of some allosteric enzymes is stimulated by specific positive modulators. An allosteric enzyme that regulates one of the catabolic reaction sequences may, for example, be subject to the stimulatory influence of positive modulators, ADP or AMP, and the inhibitory effect of negative modulator, ATP. There are also cases when an allosteric enzyme of a metabolic pathway reacts in a specific way to intermediate or final products of other metabolic pathways. Thanks to this, it is possible to coordinate the speed of action of various enzyme systems.
The second type of mechanisms that regulate metabolism in higher organisms is hormonal regulation (Fig. 13-16). Hormones are special chemical substances (chemical “messengers”) produced by various endocrine glands and released directly into the blood; they are transported by the blood to other tissues or organs and here they stimulate or inhibit certain types of metabolic activity. The hormone epinephrine, for example, is secreted by the adrenal medulla and carried by the blood to the liver, where it stimulates the breakdown of glycogen into glucose, which causes an increase in blood sugar levels. In addition, adrenaline stimulates the breakdown of glycogen in skeletal muscles; this process leads to the formation of lactate and the storage of energy in the form of ATP. Epinephrine produces these effects by attaching to specific receptor sites on the surface of muscle cells or liver cells.
The binding of adrenaline serves as a signal; this signal is transmitted to the internal parts of the cell and causes a covalent modification here, under the influence of which glycogen phosphorylase (the first enzyme in the system that catalyzes the conversion of glycogen into glucose and other products; section 9.22) passes from a less active form to a more active one (Fig. 13-16 ).
The third type of mechanisms regulating metabolism is associated with changes in the concentration of this enzyme in the cell. The concentration of any enzyme at any given moment is determined by the ratio of the rates of its synthesis and decay. The rate of synthesis of some enzymes increases sharply under certain conditions; Accordingly, the concentration of this enzyme in the cell increases. If, for example, an animal receives a diet rich in carbohydrates but poor in protein, then its liver contains extremely low levels of enzymes that under normal conditions catalyze the breakdown of amino acids to acetyl-CoA. Since these enzymes are practically not needed with such a diet, they are not produced in large quantities. It is worth, however, switching the animal to a diet rich in protein, and within a day the content of enzymes in its liver will noticeably increase, which will now be required to break down digestible amino acids.

Rice. 13-16. Hormonal regulation of enzymatic reactions. As a result of the attachment of the hormone adrenaline to specific receptors located on the surface of liver cells, cyclic adenylate is formed with the participation of a membrane-bound enzyme (adenylate cyclase). The latter functions as an allosteric activator, or intracellular mediator, under the influence of which glycogen phosphorylase passes from an inactive form to an active one, which entails an acceleration of the conversion of liver glycogen into blood glucose. This metabolic pathway is described in detail in Chap. 25.

Rice. 13-17. Enzyme induction. A high intracellular concentration of substrate A can stimulate the biosynthesis of enzymes E1, E2 and E3. The content of these enzymes in the cell increases, and thereby creates the opportunity to accelerate those reactions, as a result of which excess substrate A is removed. An excess of substrate A therefore serves as a signal for the cell nucleus, forcing it to “turn on” the genes that control the formation of the enzymes El, E2 and E3. Inclusion of genes means the synthesis of the corresponding messenger RNA; it enters the ribosomes, and as a result, the synthesis of enzymes E1, E2 and E3 takes place in them.
Liver cells, therefore, have the ability to turn on or off the biosynthesis of specific enzymes, depending on the nature of the nutrients entering them. This phenomenon is called enzyme induction (Fig. 13-17).
1. All chemical reactions in a cell occur with the participation of enzymes. Therefore, in order to influence the rate of the metabolic pathway (the sequential transformation of one substance into another), it is enough to regulate the number of enzyme molecules or their activity. Usually in metabolic pathways there are key enzymes due to which the speed of the entire path is regulated. These enzymes (one or more in a metabolic pathway) are called regulatory enzymes. Regulation of the rate of enzymatic reactions is carried out at three independent levels: by changing the number of enzyme molecules, the availability of substrate and coenzyme molecules, and changing the catalytic activity of the enzyme molecule (Table 2.6).
Table 2.5. Methods for regulating the rate of enzymatic reactions
| Method of regulation | Characteristic |
| Change in the number of enzyme molecules | The number of enzyme molecules in a cell is determined by the ratio of two processes: synthesis and decay. The most studied mechanism of regulation of enzyme synthesis is at the level of transcription (mRNA synthesis), which is regulated by certain metabolites, hormones and a number of biologically active molecules. |
| Availability of substrate and coenzyme molecules | An important parameter that controls the course of an enzymatic reaction is the presence of substrate and coenzyme. The higher the concentration of the starting substrate, the higher the reaction rate |
| Change in the catalytic activity of an enzyme molecule | The main ways to regulate enzyme activity are: - allosteric regulation; - regulation using protein-protein interactions; - regulation by phosphorylation-dephosphorylation of the enzyme molecule; - regulation by partial (limited) proteolysis |
Let's consider ways to regulate the rate of enzymatic reactions by changing the catalytic activity of the enzyme molecule.
2. Allosteric regulation. Allosteric enzymes called enzymes, activity which can be adjusted by using effector substances. The effectors involved in allosteric regulation are cellular metabolites that are often participants in the very pathway they regulate.
The effector that causes reduction (inhibition) enzyme activity is called inhibitor. The effector that causes increase (activation) enzyme activity is called activator.
Allosteric enzymes have certain structural features:
Usually are oligomeric proteins, consisting of several protomers;
Have allosteric center, spatially distant from the catalytic active site;
Effectors attach to the enzyme non-covalently at allosteric (regulatory) centers.
Allosteric centers, like catalytic ones, can exhibit different specificity with respect to ligands: it can be absolute or group. Some enzymes have several allosteric centers, some of which are specific to activators, others to inhibitors.
The protomer on which the allosteric center is located is called regulatory protomer Unlike catalytic protomer, containing an active center in which a chemical reaction takes place.
Allosteric enzymes have the property cooperativeness: the interaction of an allosteric effector with an allosteric center causes a cooperative change in the conformation of all subunits, leading to a change in the conformation of the active center and a change in the affinity of the enzyme for the substrate, which reduces or increases the catalytic activity of the enzyme. If an inhibitor is attached to the allosteric center, then as a result of cooperative conformational changes, a change in the conformation of the active center occurs, which causes a decrease in the affinity of the enzyme for the substrate and, accordingly, a decrease in the rate of the enzymatic reaction. Conversely, if an activator is attached to the allosteric center, then the affinity of the enzyme for the substrate increases, which causes an increase in the reaction rate. The sequence of events under the action of allosteric effectors is presented in Fig. 2.26.
Regulation of allosteric enzymes reversible: detachment of the effector from the regulatory subunit restores the original catalytic activity of the enzyme.
Allosteric enzymes catalyze key reactions of this metabolic pathway.
Allosteric enzymes play an important role in various metabolic pathways, as they respond extremely quickly to the slightest changes in the internal composition of the cell. The speed of metabolic processes depends on the concentration of substances, both used and formed in a given chain of reactions. Precursors can be activators of allosteric enzymes in the metabolic pathway. At the same time, when the end product of any metabolic pathway accumulates, it can act as an allosteric inhibitor of the enzyme. This method of regulation is common in the body and is called “negative feedback”:
 Rice. 2.26. Scheme of the structure and functioning of an allosteric enzyme:
Rice. 2.26. Scheme of the structure and functioning of an allosteric enzyme:
A - the action of a negative effector (inhibitor). The inhibitor (I) attaches to the allosteric center, which causes cooperative conformational changes in the enzyme molecule, including in the active center of the enzyme. The affinity of the enzyme for the substrate decreases, and as a result, the rate of the enzymatic reaction decreases; B - action of a positive effector (activator). The activator (A) binds to the allosteric center, which causes cooperative conformational changes. The affinity of the enzyme for the substrate increases and the rate of the enzymatic reaction increases. The reversible effect of both inhibitor and activator on enzyme activity has been demonstrated
Let's consider the allosteric regulation of the process of glucose catabolism, which ends with the formation of an ATP molecule (Fig. 2.27). In the event that ATP molecules in the cell are not consumed, it is an inhibitor of allosteric enzymes of this metabolic pathway: phosphofructokinase and pyruvate kinase. At the same time, the intermediate metabolite of glucose catabolism, fructose-1,6-bisphosphate, is an allosteric activator of the pyruvate kinase enzyme. Inhibition by the end product of the metabolic pathway and activation by the initial metabolites allows
 Rice. 2.27. Allosteric regulation of the process of glucose catabolism.
Rice. 2.27. Allosteric regulation of the process of glucose catabolism.
The ATP molecule is an allosteric inhibitor of metabolic pathway enzymes - phosphofructokinase and pyruvate kinase. The fructose-1,6-bisphosphate molecule is an allosteric activator of the enzyme pyruvate kinase
regulate the speed of the metabolic pathway. Allosteric enzymes catalyze, as a rule, the initial reactions of a metabolic pathway, irreversible reactions, rate-limiting reactions (the slowest) or reactions at the branch point of a metabolic pathway.
3. Regulation by protein-protein interactions. Some enzymes change their activity as a result of protein-protein interactions. At least two mechanisms for changing enzyme activity in this way can be distinguished: activation of enzymes as a result of the addition of activator proteins (activation of the enzyme adenylate cyclase by the α-subunit of the G protein, see module 4) and changes in catalytic activity as a result of association and dissociation of protomers.
As an example of the regulation of the catalytic activity of enzymes by association or dissociation of protomers, we can consider the regulation of the enzyme protein kinase A.
Protein kinase A(cAMP-dependent) consists of four subunits of two types: two regulatory (R) and two catalytic (C). This tetramer does not have catalytic activity. Regulatory subunits have binding sites for cyclic 3",5"-AMP (cAMP) (two for each subunit). The attachment of four cAMP molecules to two regulatory subunits leads to a change in the conformation of the regulatory protomers and to the dissociation of the tetrameric complex; this releases two active catalytic subunits (Fig. 2.28). Active protein kinase A catalyzes the transfer of a phosphoric acid residue from ATP to specific OH groups of amino acid residues of proteins (i.e., it causes phosphorylation of proteins).
 Rice. 2.28. Regulation of protein kinase A (PKA) activity by protein-protein interactions.
Rice. 2.28. Regulation of protein kinase A (PKA) activity by protein-protein interactions.
PKA is activated by four cAMP molecules, which bind to two regulatory subunits, which leads to a change in the conformation of the regulatory protomers and dissociation of the tetrameric complex. This releases two active catalytic subunits that can cause protein phosphorylation
The cleavage of cAMP molecules from the regulatory subunits leads to the association of the regulatory and catalytic subunits of proten kinase A with the formation of an inactive complex.
4. Regulation of the catalytic activity of enzymes by phosphorylation-dephosphorylation. In biological systems, a mechanism for regulating the activity of enzymes using their covalent modification is often found. A quick and widespread method of chemical modification of enzymes is their phosphorylation-dephosphorylation.
The OH groups of the enzyme undergo phosphorylation, which is carried out by enzymes protein kinases(phosphorylation) and phosphoprotein phosphatases(dephosphorylation). The addition of a phosphoric acid residue leads to a change in the conformation of the active center and its catalytic activity. In this case, the result can be twofold: some enzymes are activated during phosphorylation, while others, on the contrary, become less active (Fig. 2.29). The activity of protein kinases and phosphoprotein phosphatases is regulated by hormones, which allows the activity of key enzymes in metabolic pathways to rapidly vary depending on environmental conditions.
 Rice. 2.29. Scheme of regulation of enzyme activity by phosphorylation-dephosphorylation.
Rice. 2.29. Scheme of regulation of enzyme activity by phosphorylation-dephosphorylation.
Phosphorylation of enzymes occurs with the help of the enzyme protein kinase. The donor of the phosphoric acid residue is the ATP molecule. Phosphorylation of an enzyme changes its conformation and the conformation of the active site, which changes the affinity of the enzyme for the substrate. In this case, some enzymes are activated during phosphorylation, while others are inhibited. The reverse process - dephosphorylation - is caused by the enzymes phosphoprotein phosphatases, which cleave off the phosphoric acid residue from the enzyme and return the enzyme to its original state
5. Regulation of the catalytic activity of enzymes by partial (limited) proteolysis. Some enzymes that function outside cells (in the gastrointestinal tract or blood plasma) are synthesized as inactive precursors and are activated only as a result of hydrolysis of one or more specific peptide bonds, which leads to the elimination of part of the molecule. In the remaining part of the protein molecule, a conformational rearrangement occurs and the active center of the enzyme is formed (Fig. 2.30). Partial proteolysis is an example of regulation when the activity of an enzyme is changed
 Rice. 2.30. Activation of pepsin by partial proteolysis.
Rice. 2.30. Activation of pepsin by partial proteolysis.
As a result of hydrolysis of one or more peptide bonds of pepsinogen (an inactive molecule), part of the molecule is split off and the active center of the pepsin enzyme is formed
irreversible. Such enzymes usually function for a short time, determined by the lifetime of the protein molecule. Partial proteolysis underlies the activation of digestive proteolytic enzymes (pepsin, trypsin, chymotrypsin, elastase), peptide hormones (insulin), proteins of the blood coagulation system and a number of other proteins.