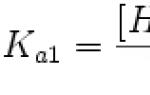Potassium. Properties of potassium. Uses of potassium Where is potassium found in nature?
Potassium is an element of the main subgroup of the first group, the fourth period of the periodic table of chemical elements, with atomic number 19. Denoted by the symbol K (lat. Kalium). The simple substance potassium (CAS number: 7440-09-7) is a soft alkali metal of silvery-white color.
In nature, potassium is found only in combination with other elements, for example, in sea water, as well as in many minerals. It oxidizes very quickly in air and very easily enters into chemical reactions, especially with water, forming an alkali. In many respects, the chemical properties of potassium are very similar to sodium, but in terms of biological function and use by the cells of living organisms, they are still different.
History and origin of the name
Potassium (more precisely, its compounds) has been used since ancient times. Thus, the production of potash (which was used as a detergent) existed already in the 11th century. The ash formed when burning straw or wood was treated with water, and the resulting solution (lye) was evaporated after filtering. The dry residue, in addition to potassium carbonate, contained potassium sulfate K2SO4, soda and potassium chloride KCl.
In 1807, the English chemist Davy isolated potassium by electrolysis of molten potassium hydroxide (KOH) and named it “potassium” (Latin potassium; this name is still used in English, French, Spanish, Portuguese and Polish). In 1809, L. V. Gilbert proposed the name “potassium” (Latin kalium, from Arabic al-kali - potash). This name entered the German language, from there into most languages of Northern and Eastern Europe (including Russian) and “won” when choosing the symbol for this element - K.
Receipt
Potassium, like other alkali metals, is obtained by electrolysis of molten chlorides or alkalis. Since chlorides have a higher melting point (600-650 °C), electrolysis of straightened alkalis is more often carried out with the addition of soda or potash (up to 12%). During the electrolysis of molten chlorides, molten potassium is released at the cathode, and chlorine is released at the anode:
K + + e - → K
2Cl - − 2e - → Cl 2
During the electrolysis of alkalis, molten potassium is also released at the cathode, and oxygen is released at the anode:
4OH - − 4e - → 2H 2 O + O 2
The water from the melt evaporates quickly. To prevent potassium from interacting with chlorine or oxygen, the cathode is made of copper and a copper cylinder is placed above it. The resulting potassium is collected in molten form in a cylinder. The anode is also made in the form of a cylinder of nickel (for the electrolysis of alkalis) or of graphite (for the electrolysis of chlorides).
Physical properties
Potassium is a silvery substance with a characteristic shine on a freshly formed surface. Very light and fusible. It dissolves relatively well in mercury, forming amalgams. When potassium (as well as its compounds) is added to the burner flame, it colors the flame in a characteristic pink-violet color.
Chemical properties
Elemental potassium, like other alkali metals, exhibits typical metallic properties and is very chemically active and a strong reducing agent. In air, a fresh cut quickly fades due to the formation of films of compounds (oxides and carbonate). With prolonged contact with the atmosphere it can completely collapse. Reacts explosively with water. It must be stored under a layer of gasoline, kerosene or silicone to prevent contact of air and water with its surface. Potassium forms intermetallic compounds with Na, Tl, Sn, Pb, Bi.
Potassium compounds, like its closest chemical analogue - sodium, have been known since ancient times and found application in various areas of human activity. However, these metals themselves were first isolated in a free state only in 1807 during experiments by the English. scientist G. Davy. By electrolysis of slightly moistened solid alkalis, free metals - potassium and sodium - were obtained. Davy called the new metal Potassium, but this name did not stick.
The godfather of metal turned out to be Gilbert, the famous publisher of the magazine "Annalen de Physik", who proposed the name "potassium"; it was adopted in Germany and Russia. Both names come from terms that were used long before the discovery of potassium metal.
The word potassium is derived from the word potash, which probably appeared in the 16th century. It is found in Van Helmont in the second half of the 17th century. is widely used as the name of a commercial product - potash - in Russia, England and Holland. Translated into Russian, the word potashe means “pot ash or ash boiled in a pot”; in the XVI - XVII centuries. potash was obtained in huge quantities from wood ash, which was boiled in large boilers. Potash was used to prepare mainly literated (purified) saltpeter, which was used to make gunpowder. Especially a lot of potash was produced in Russia, in the forests near Arzamas and Ardatov in mobile factories (Maidans) that belonged to a relative of Tsar Alexei Mikhailovich, a close boyar B.I. Morozov.
As for the word potassium, it comes from the Arabic term alkali (alkaline substances). In the Middle Ages, alkalis, or, as they said then, alkaline salts, were almost indistinguishable from each other and were called by names that had the same meaning: natron, borax, varek, etc. The word kali (qila) was found around 850 Arab writers, then the word Qali (al-Qali) began to be used, which denoted a product obtained from the ash of some plants; the Arabic qiljin or qaljan (ash) and qalaj (burn) are associated with these words. In the era of atrochemistry, alkalis began to be divided into “fixed” and “volatile”. In the 17th century There are names alkali fixum minerale (mineral fixed alkali or caustic soda), alkali fixum. vegetabile (vegetable fixed alkali or potash and caustic potassium), as well as alkali volatile (volatile alkali or NH3). Black established a distinction between caustic and soft, or carbonic, alkalis. Alkalies do not appear in the Table of Simple Bodies, but in a note to the table Lavoisier indicates that the fixed alkalis (potash and soda) are probably complex substances, although the nature of their constituent parts has not yet been studied. In Russian chemical literature of the first quarter of the 19th century. potassium was called potassium (Soloviev, 1824), potash (Strakhovoy, 1825), potash (Shcheglov, 1830); in the "Dvigubsky Store" already in 1828, along with the name potash (potash sulfate), the name potassium (caustic potassium, salt potassium, etc.) is found. The name potassium became generally accepted after the publication of Hess's textbook.
| Atomic number | |
| Appearance of a simple substance |
Silver-white soft metal |
| Properties of the atom | |
| Atomic mass (molar mass) |
39.0983 a. e.m. (g/mol) |
| Atomic radius | |
| Ionization energy (first electron) |
418.5 (4.34) kJ/mol (eV) |
| Electronic configuration | |
| Chemical properties | |
| Covalent radius | |
| Ion radius | |
| Electronegativity (according to Pauling) |
|
| Electrode potential | |
| Oxidation states | |
| Thermodynamic properties of a simple substance | |
| Density | |
| Molar heat capacity |
29.6 J/(K mol) |
| Thermal conductivity |
79.0 W/(m K) |
| Melting temperature | |
| Heat of Melting |
102.5 kJ/mol |
| Boiling temperature | |
| Heat of vaporization |
2.33 kJ/mol |
| Molar volume |
45.3 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure |
cubic body-centered |
| Lattice parameters | |
| c/a ratio | — |
| Debye temperature | |
| K | 19 |
| 39,0983 | |
| 4s 1 | |
- an element of the main subgroup of the first group, the fourth period of the periodic system of chemical elements of D.I. Mendeleev, with atomic number 19. Denoted by the symbol K (lat. Kalium). The simple substance potassium (CAS number: 7440-09-7) is a soft alkali metal with a silvery-white color. In nature, potassium is found only in combination with other elements, for example, in sea water, as well as in many minerals. It oxidizes very quickly in air and very easily enters into chemical reactions, especially with water, forming an alkali. In many respects, the chemical properties of potassium are very similar to sodium, but in terms of biological function and use by the cells of living organisms, they are still different. History and origin of the name potassium
Potassium (more precisely, its compounds) has been used since ancient times. Thus, the production of potash (which was used as a detergent) existed already in the 11th century. The ash formed when burning straw or wood was treated with water, and the resulting solution (lye) was evaporated after filtering. The dry residue, in addition to potassium carbonate, contained potassium sulfate K2SO4, soda and potassium chloride KCl.
In 1807, the English chemist Davy isolated potassium by electrolysis of solid potassium hydroxide (KOH) and named it "potassian"(lat. potassium; this name is still used in English, French, Spanish, Portuguese and Polish). In 1809, L. V. Gilbert proposed the name “potassium” (lat. kalium, from Arabic. al-kali - potash). This name entered the German language, from there into most languages of Northern and Eastern Europe (including Russian) and “won” when choosing a symbol for this element - K.
Presence of potassium in nature
Not found in a free state. Potassium is part of sylvinite KCl NaCl, carnallite KCl MgCl 2 6H 2 O, kainite KCl MgSO 4 6H 2 O, and is also present in the ash of some plants in the form of carbonate K 2 CO 3 (potash). Potassium is found in all cells (see section below Biological role).
Potassium - getting potassium
Potassium, like other alkali metals, is obtained by electrolysis of molten chlorides or alkalis. Since chlorides have a higher melting point (600-650 °C), electrolysis of straightened alkalis is more often carried out with the addition of soda or potash (up to 12%). During the electrolysis of molten chlorides, molten potassium is released at the cathode, and chlorine is released at the anode:
K + + e − → K
2Cl − − 2e − → Cl 2
During the electrolysis of alkalis, molten potassium is also released at the cathode, and oxygen is released at the anode:
4OH − − 4e − → 2H 2 O + O 2
The water from the melt evaporates quickly. To prevent potassium from interacting with chlorine or oxygen, the cathode is made of copper and a copper cylinder is placed above it. The resulting potassium is collected in molten form in a cylinder. The anode is also made in the form of a cylinder of nickel (for the electrolysis of alkalis) or of graphite (for the electrolysis of chlorides).
Physical properties of potassium
Potassium is a silvery substance with a characteristic shine on a freshly formed surface. Very light and fusible. It dissolves relatively well in mercury, forming amalgams. When potassium (as well as its compounds) is added to the burner flame, it colors the flame in a characteristic pink-violet color.
Chemical properties of potassium
Potassium, like other alkali metals, exhibits typical metallic properties and is very chemically active, easily donating electrons.
Is a strong reducing agent. It combines so actively with oxygen that not an oxide is formed, but potassium superoxide KO 2 (or K 2 O 4). When heated in a hydrogen atmosphere, potassium hydride KH is formed. It interacts well with all non-metals, forming halides, sulfides, nitrides, phosphides, etc., as well as with complex substances such as water (the reaction occurs explosively), various oxides and salts. In this case, they reduce other metals to a free state.
Potassium is stored under a layer of kerosene.
Potassium oxides and potassium peroxides
When potassium reacts with atmospheric oxygen, it forms not an oxide, but a peroxide and superoxide:
Potassium oxide can be obtained by heating the metal to a temperature not exceeding 180 °C in an environment containing very little oxygen, or by heating a mixture of potassium superoxide with potassium metal:
Potassium oxides have pronounced basic properties and react violently with water, acids and acid oxides. They have no practical significance. Peroxides are yellowish-white powders that, soluble in water, form alkalis and hydrogen peroxide:
The ability to exchange carbon dioxide for oxygen is used in insulating gas masks and on submarines. An equimolar mixture of potassium superoxide and sodium peroxide is used as an absorber. If the mixture is not equimolar, then in the case of an excess of sodium peroxide, more gas will be absorbed than released (when absorbing two volumes of CO 2, one volume of O 2 is released), and the pressure in a confined space will drop, and in the case of an excess of potassium superoxide (when absorbing two volumes of CO 2 three volumes of O are released 2) more gas is released than absorbed, and the pressure will increase.
In the case of an equimolar mixture (Na 2 O 2:K 2 O 4 = 1:1), the volumes of absorbed and released gases will be equal (when four volumes of CO 2 are absorbed, four volumes of O 2 are released).
Peroxides are strong oxidizing agents, so they are used to bleach fabrics in the textile industry.
Peroxides are obtained by calcining metals in air freed from carbon dioxide.
Potassium hydroxides
Potassium hydroxide (or caustic potassium) are hard white opaque, very hygroscopic crystals that melt at a temperature of 360 °C. Potassium hydroxide is an alkali. It dissolves well in water and releases a large amount of heat. The solubility of potassium hydroxide at 20 °C in 100 g of water is 112 g.
Potassium uses
- An alloy of potassium and sodium, liquid at room temperature, is used as a coolant in closed systems, for example, in fast neutron nuclear power plants. In addition, its liquid alloys with rubidium and cesium are widely used. The alloy of composition sodium 12%, potassium 47%, cesium 41% has a record low melting point of −78 °C.
- Potassium compounds are the most important biogenic element and are therefore used as fertilizers.
- Potassium salts are widely used in electroplating because, despite their relatively high cost, they are often more soluble than the corresponding sodium salts, and therefore provide intensive operation of electrolytes at increased current densities.
Important Connections
Purple color of potassium ions flame in burner flame
- Potassium bromide is used in medicine and as a sedative for the nervous system.
- Potassium hydroxide (caustic potash) - used in alkaline batteries and when drying gases.
- Potassium carbonate (potash) - used as fertilizer in glass making.
- Potassium chloride (sylvin, "potassium salt") - used as a fertilizer.
- Potassium nitrate (potassium nitrate) is a fertilizer, a component of black powder.
- Potassium perchlorate and chlorate (Bertholet salt) are used in the production of matches, rocket powders, lighting charges, explosives, and in electroplating.
- Potassium dichromate (chrompic) is a strong oxidizing agent, used to prepare a “chromium mixture” for washing chemical dishes and in leather processing (tanning). It is also used to purify acetylene in acetylene plants from ammonia, hydrogen sulfide and phosphine.
- Potassium permanganate is a strong oxidizing agent, used as an antiseptic in medicine and for the laboratory production of oxygen.
- Sodium potassium tartrate (Rochelle salt) as a piezoelectric.
- Potassium dihydrogen phosphate and dideuterophosphate in the form of single crystals in laser technology.
- Potassium peroxide and potassium superoxide are used for air regeneration in submarines and in insulating gas masks (absorbs carbon dioxide to release oxygen).
- Potassium fluoroborate is an important flux for soldering steels and non-ferrous metals.
- Potassium cyanide is used in electroplating (silvering, gilding), gold mining and nitrocarburizing of steel.
- Potassium, together with potassium peroxide, is used in the thermochemical decomposition of water into hydrogen and oxygen (potassium cycle "Gaz de France", France).
Biological role
Potassium is the most important biogenic element, especially in the plant world. If there is a lack of potassium in the soil, plants develop very poorly, the yield decreases, therefore about 90% of the extracted potassium salts are used as fertilizers.
Potassium in the human body
Potassium is found mostly in cells, up to 40 times more than in the intercellular space. As cells function, excess potassium leaves the cytoplasm, so to maintain concentration it must be pumped back through the sodium-potassium pump.
Potassium and sodium are functionally related to each other and perform the following functions:
- Creating conditions for the occurrence of membrane potential and muscle contractions.
- Maintaining blood osmotic concentration.
- Maintaining acid-base balance.
- Normalization of water balance.
- Ensuring membrane transport.
- Activation of various enzymes.
- Normalization of heart rhythm.
The recommended daily dose of potassium is from 600 to 1700 milligrams for children, and from 1800 to 5000 milligrams for adults. The need for potassium depends on total body weight, physical activity, physiological state, and climate of the place of residence. Vomiting, prolonged diarrhea, profuse sweating, and the use of diuretics increase the body's need for potassium.
The main food sources are dried apricots, melon, beans, kiwi, potatoes, avocados, bananas, broccoli, liver, milk, nut butters, citrus fruits, grapes. There is a lot of potassium in fish and dairy products.
Absorption occurs in the small intestine. The absorption of potassium is facilitated by vitamin B6, and complicated by alcohol.
With a lack of potassium, hypokalemia develops. Disturbances in the functioning of the cardiac and skeletal muscles occur. Long-term potassium deficiency can cause acute neuralgia.
Potassium is an elemental substance, a metal, so active that it does not occur in nature in the form of nuggets. Potassium is included in minerals and sea water, in the organisms of plants and animals, and ranks 7th in abundance. It is of great biogenic importance, as it is necessary for the functioning of living cells.
Physical and chemical properties of potassium
Potassium is a soft substance (can be cut with a knife), silvery in color, light (lighter than water), fusible. Burns with a pink-violet flame.
An alkali metal that reacts actively with oxygen, water, halogens, and dilute acids; reactions are often accompanied by an explosion. Does not react with nitrogen. Reacts with alkalis and alcohols.
Working with pure potassium requires the use of protective equipment, since contact with even the smallest particles on the skin or eyes causes serious burns.
Potassium should be stored in sealed iron vessels under a layer of substances that prevent contact with air: mineral oil, silicone, dehydrated kerosene.
Use of potassium and its compounds
In the form of pure metal, the substance is used in a limited range of areas:
- electrodes in some current sources are made from it;
- used in electron tubes as a gas adsorbent that maintains a vacuum; in photocells, in gas-discharge lamps and devices, in thermionic converters, in photomultipliers;
- for the production of superoxide;
- using the potassium-40 isotope, the age of rocks is calculated;
- artificial isotope potassium-42 is used as a radioactive tracer in medicine and biology;
- an alloy of potassium and sodium - a liquid substance under normal conditions, used as a coolant in nuclear reactors. Other liquid potassium alloys are also used.
 Various potassium compounds are much more in demand.
Various potassium compounds are much more in demand.
- In medical practice, potassium chloride, potassium iodide, permanganate, and potassium bromide are used. Potassium is necessarily included in complex vitamin-mineral preparations. Our body needs it for muscle function, including the heart; to maintain a balanced blood composition, water and acid-base balance.
- The lion's share of potassium obtained by industry (more than 90%) goes to the production of potash fertilizers, which are vital for plant development. For this purpose, various potassium salts are used in agriculture. The most popular is the potassium salt of nitric acid, known as potassium nitrate, Indian or potassium nitrate.
- KOH (potassium hydroxide) is used in batteries to dry gases.
- Potash (potassium carbonate) is used to produce potash optical glass, in the production of fertilizers, in the processes of gas purification, drying, and tanning leather. 
- Potassium peroxide and superoxide absorb carbon dioxide and release oxygen. This property is used to regenerate oxygen in gas masks, in mines, on submarines, and in spaceships.
- Fabrics are bleached using peroxides.
- Potassium compounds are part of various explosives and flammable substances.
- Potassium permanganate is used for laboratory production of O2.
- Potassium compounds are used in electroplating and organic synthesis, in laser technology and photography, in the production of acetylene and steels and piezoelectronics. They are used for soldering non-ferrous metals and steels, and for washing chemical utensils.
Potassium iodide, potassium nitrate, potassium carbonate are only a small part of the potassium compounds that our chemical reagents store offers. In Moscow and the Moscow region, purchasing goods for laboratories and production from Prime Chemicals Group is convenient and profitable. We have excellent service, delivery and pickup options.
POTASSIUM (Latin Kalium), K, chemical element of group I of the short form (group 1 of the long form) of the periodic system; atomic number 19; atomic mass 39.0983; refers to alkali metals. Natural potassium consists of three isotopes: 39 K (93.2581%), 40 K (0.0117%; weakly radioactive, T 1/2 1.277 10 9 years, β-decay up to 40 Ca), 41 K (6.7302 %). Radioisotopes with mass numbers 32-54 have been artificially obtained.
Historical reference. Some potassium compounds were known in ancient times, for example, potassium carbonate K 2 CO 3 (the so-called plant alkali) was isolated from wood ash and used in making soap. Metallic potassium was first obtained by G. Davy in 1807 by electrolysis of wet solid KOH hydroxide and named potassium (English potassium from English potash - the name of potassium carbonate). In 1809, the name “potassium” (from the Arabic al-kali - potash) was proposed. The name “potassium” has been preserved in Great Britain, the USA, France and other countries. In Russia, since 1840, the name “potassium” has been used, also adopted in Germany, Austria, and the Scandinavian countries.
Prevalence in nature. The potassium content in the earth's crust is 2.6% by weight. Potassium does not occur in a free state in nature. Potassium is found in significant quantities in nepheline and leucite silicates, feldspars (for example, orthoclase), and micas (for example, muscovite). Own potassium minerals - sylvite, sylvinite, carnallite, kainite, langbeinite K 2 SO 4 ∙2MgSO 4 form large accumulations of natural potassium salts. As a result of the action of water and carbon dioxide, potassium turns into soluble compounds, which are partially carried into the seas and partially retained by the soil. Potassium salts are also found in the brine of salt lakes and underground brines.
Properties. The configuration of the outer electron shell of the potassium atom is 4s 1; in compounds exhibits an oxidation state of +1; ionization energies K 0 →K + →K 2+ are respectively 4.3407 and 31.8196 eV; Pauling electronegativity 0.82; atomic radius 220 pm, radius of the K + ion 152 pm (coordination number 6).
Potassium is a silvery-white soft metal; body-centered cubic crystal lattice; t melt 63.38 °C, t boil 759 °C, density 856 kg/m 3 (20 °C); heat capacity 29.60 J/(mol K) at 298 K.
Potassium can be pressed and rolled, easily cut with a knife and retains plasticity at low temperatures; Brinell hardness 0.4 MPa.
Potassium is a metal of high chemical activity (potassium is stored under a layer of gasoline, kerosene or mineral oil). Under normal conditions, potassium interacts with oxygen (K 2 O oxide, K 2 O 2 peroxide, superoxide KO 2 is formed - the main product), halogens (corresponding potassium halides), when heated - with sulfur (K 2 S sulfide), selenium (selenide K 2 Se), tellurium (K 2 Te telluride), with phosphorus in a nitrogen atmosphere (phosphides K 3 P and K 2 P5), carbon (layered compounds of the composition KS 8 - KS 60), hydrogen (KN hydride). Potassium interacts with nitrogen only when exposed to an electric discharge (KN 3 azide and K 3 N nitride are formed in small quantities). Potassium reacts with some metals, forming intermetallic compounds or solid solutions (potassium alloys). Alloys with sodium, characterized by high chemical activity, are of greatest practical importance; obtained by alloying metals in an inert atmosphere or by the action of metallic sodium on KOH hydroxide or KCl chloride.
Potassium metal is a strong reducing agent: it reacts vigorously (under normal conditions with explosion and ignition of the metal) with water (potassium hydroxide KOH is formed), reacts violently (sometimes with explosion) with acids (the corresponding salts are formed, for example potassium dichromate, potassium nitrate, potassium permanganate, potassium phosphates, potassium cyanide), reduces oxides of B, Si, Al, Ag, Bi, Co, Cr, Cu, Hg, Ni, Pb, Sn, Ti to elements; sulfates, sulfites, nitrates, nitrites, carbonates and phosphates of other metals - to oxides of the corresponding metals. Potassium metal dissolves slowly in liquid ammonia to form a dark blue solution with metallic conductivity; the dissolved metal gradually reacts with ammonia to form an amide: 2K + 2NH 3 = 2KNH 2 + H 2. Potassium interacts with various organic compounds: alcohols (alcoholates are formed, for example ethylate C 2 H 5 OK), acetylene (acetylenides KS≡CH and KS≡SK), alkyl halides (potassium alkyls, for example ethylpotassium C 2 H 5 K) and aryl halides (potassium aryls, for example phenylpotassium C 6 H 5 K). Potassium metal initiates the polymerization reactions of alkenes and dienes. With N- and O-donor polycyclic ligands (crown ethers, cryptands and other ionophores), potassium forms complex compounds.
When working with potassium, it is necessary to take into account its high reactivity, including the ability to ignite upon contact with water. For safety reasons, you must use rubber gloves, safety glasses or a mask. Large amounts of potassium should be worked in special chambers in an inert atmosphere (argon, nitrogen). To extinguish burning potassium, use table salt NaCl or soda ash Na 2 CO 3.
Biological role. Potassium is a biogenic element. The daily human need for potassium is about 2 g. In living organisms, potassium ions play an important role in the processes of regulating metabolism, in particular, the transport of ions through cell membranes (see, for example, the article Ion pumps).
Receipt. In industry, potassium is obtained by reducing molten KOH hydroxide or KCl chloride with sodium metal in a countercurrent column, followed by condensation of potassium vapor. Vacuum-thermal methods for producing potassium are promising, based on the reduction of KCl chloride when heated with a mixture of aluminum or silicon with calcium oxide (6Kl + 2Al + 4CaO = 6K + 3CaCl 2 + CaO Al 2 O 3 or 4Kl + Si + 4CaO = 4K + 2CaCl 2 + 2CaO∙SiO 2), as well as a method based on the production of a potassium alloy with lead by electrolysis of K 2 CO 3 carbonate or KCl chloride with a molten lead cathode and subsequent distillation of the potassium alloy. The volume of global potassium production is about 28 tons/year (2004).
Application. Metallic potassium is a material for electrodes in chemical power sources, a catalyst in the processes of producing synthetic rubber. Various potassium compounds are widely used: peroxide K 2 O 2 and superperoxide KO 2 - components of compositions for oxygen regeneration (in submarines, spacecraft and other enclosed spaces), KN hydride - a reducing agent in chemical synthesis, an alloy of potassium with sodium (10- 60% Na by weight, liquid at room temperature) - a coolant in nuclear reactors, a reducing agent in titanium production, a reagent for purifying gases from oxygen and water vapor; Potassium salts are used as potassium fertilizers and components of detergents. Complexes of potassium with ionophores are models for studying the transport of potassium ions across cell membranes. The radioisotope 42 K (T 1/2 12.36 h) is used as a radioactive indicator in chemistry, medicine and biology.
Lit.: Sodium and potassium. L., 1959; Stepin B. D., Tsvetkov A. A. Inorganic chemistry. M., 1994; Inorganic chemistry: chemistry of elements / Edited by Yu. D. Tretyakov. M., 2004. T. 2.