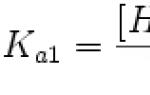What is the difference between primary and secondary X-rays? X-rays. Application of X-rays in medicine
1. Bremsstrahlung and characteristic X-ray radiation,
main properties and characteristics.
In 1895, the German scientist Roentgen first discovered the glow of a fluorescent screen, which was caused by radiation invisible to the eye coming from the glass section of the gas discharge tube located opposite the cathode. This type of radiation had the ability to pass through substances impenetrable to visible light. Roentgen called them X-rays and established the basic properties that allow them to be used in various branches of science and technology, including medicine.
X-ray radiation is radiation with a wavelength of 80-10 -5 nm. Long-wave X-ray radiation overlaps short-wave UV radiation, short-wave radiation overlaps long-wave g-radiation. In medicine, X-ray radiation with a wavelength from 10 to 0.005 nm is used, which corresponds to a photon energy from 10 2 EV to 0.5 MeV. X-ray radiation is invisible to the eye, so all observations with it are made using fluorescent screens or photographic films, since it causes x-ray luminescence and has a photochemical effect. It is characteristic that most bodies that are impenetrable to optical radiation are largely transparent to x-ray radiation, which has properties common to electromagnetic waves. However, due to the short wavelength, some properties are difficult to detect. Therefore, the wave nature of the radiation was established much later than their discovery.
Based on the method of excitation, X-ray radiation is divided into bremsstrahlung and characteristic radiation.
Bremsstrahlung X-rays are caused by the deceleration of fast-moving electrons by the electric field of the atom (nucleus and electrons) of the substance through which they fly. The mechanism of this radiation can be explained by the fact that any moving charge represents a current around which a magnetic field is created, the induction (B) of which depends on the speed of the electron. When braking, magnetic induction decreases and, in accordance with Maxwell's theory, an electromagnetic wave appears.
When electrons are decelerated, only part of the energy is used to create an x-ray photon, the other part is spent on heating the anode. The frequency (wavelength) of the photon depends on the initial kinetic energy of the electron and the intensity of its deceleration. Moreover, even if the initial kinetic energy is the same, then the conditions of deceleration in the substance will be different, therefore the emitted photons will have the most diverse energies, and, consequently, wavelengths, i.e. the X-ray spectrum will be continuous. Figure 1 shows the spectrum of X-ray bremsstrahlung at different voltages U 1
 .
.
If U is expressed in kilovolts and the relationship between other quantities is taken into account, then the formula looks like: l k = 1.24/U (nm) or l k = 1.24/U (Å) (1 Å = 10 -10 m).
From the above graphs it can be established that the wavelength l m, which accounts for the maximum radiation energy, is in a constant relationship with the cutoff wavelength l k:
![]() .
.
Wavelength characterizes the energy of a photon, on which the penetrating ability of radiation when it interacts with matter depends.
Short-wave X-rays usually have high penetrating power and are called hard, while long-wave X-rays are called soft. As can be seen from the above formula, the wavelength at which the maximum radiation energy occurs is inversely proportional to the voltage between the anode and cathode of the tube. By increasing the voltage at the anode of the X-ray tube, the spectral composition of the radiation is changed and its hardness increases.
When the filament voltage changes (the filament temperature of the cathode changes), the number of electrons emitted by the cathode per unit time changes, or, accordingly, the current strength in the tube anode circuit changes. In this case, the radiation power changes in proportion to the first power of the current strength. The spectral composition of the radiation will not change.
The total flux (power) of radiation, the distribution of energy over wavelengths, as well as the spectrum boundary on the side of short wavelengths depends on the following three reasons: the voltage U accelerating electrons and applied between the anode and cathode of the tube; the number of electrons involved in the formation of radiation, i.e. tube filament current; atomic number Z of the anode substance in which electron deceleration occurs.
The X-ray bremsstrahlung flux is calculated using the formula: , where ![]() ,
,
Z-atomic number of a substance (atomic number).
By increasing the voltage on the X-ray tube, one can notice the appearance of individual lines (line spectrum) against the background of continuous bremsstrahlung X-ray radiation, which corresponds to characteristic X-ray radiation. It occurs during the transition of electrons between the inner shells of atoms in a substance (shells K, L, M). The line nature of the spectrum of characteristic radiation arises due to the fact that accelerated electrons penetrate deep into the atoms and knock out electrons from their inner layers outside the atom. Electrons (Fig. 2) from the upper layers move to free places, as a result of which X-ray photons are emitted with a frequency corresponding to the difference in transition energy levels. The lines in the spectrum of characteristic radiation are combined into series corresponding to transitions of electrons with a higher level at the K, L, M level.
The external influence, as a result of which the electron is knocked out of the inner layers, must be quite strong. In contrast to optical spectra, the characteristic X-ray spectra of different atoms are of the same type. The uniformity of these spectra is due to the fact that the inner layers of different atoms are identical and differ only in energy, because the force impact from the core increases as the ordinal number of the element increases. This leads to the fact that the characteristic spectra shift towards higher frequencies with increasing nuclear charge. This relationship is known as Moseley's law: ![]() , where A and B are constants; Z-ordinal number of the element.
, where A and B are constants; Z-ordinal number of the element.
There is another difference between X-ray and optical spectra. The characteristic spectrum of an atom does not depend on the chemical compound into which the atom is included. For example, the X-ray spectrum of the oxygen atom is the same for O, O 2, H 2 O, while the optical spectra of these compounds are significantly different. This feature of the X-ray spectra of atoms served as the basis for the name “characteristic”.
Characteristic radiation occurs whenever there are free spaces in the inner layers of the atom, regardless of the reasons that caused it. For example, it accompanies one type of radioactive decay, which involves the capture of an electron from the inner layer by the nucleus.
2. Arrangement of X-ray tubes and protozoa
X-ray machine.
The most common source of X-ray radiation is an X-ray tube - a two-electrode vacuum device (Fig. 3). It is a glass balloon (p = 10 -6 – 10 -7 mm Hg) with two electrodes - anode A and cathode K, between which a high voltage is created. The heated cathode (K) emits electrons. Anode A is often called the anticathode. It has an inclined surface in order to direct the resulting X-ray radiation at an angle to the axis of the tube. The anode is made of a metal with good thermal conductivity (copper) to remove the heat generated when electrons strike. At the beveled end of the anode there is a plate 3 of a refractory metal (tungsten) with a high atomic number, called the anode mirror. In some cases, the anode is specially cooled with water or oil. For diagnostic tubes, the precision of the X-ray source is important, which can be achieved by focusing the electrons in one place on the anode. Therefore, constructively it is necessary to take into account two opposing tasks: on the one hand, electrons must fall on one place of the anode, on the other hand, in order to prevent overheating, it is desirable to distribute electrons over different areas of the anode. For this reason, some X-ray tubes are manufactured with a rotating anode.
In a tube of any design, electrons, accelerated by the voltage between the anode and the cathode, fall on the anode mirror and penetrate deep into the substance, interact with atoms and are inhibited by the field of atoms. This produces bremsstrahlung X-ray radiation. Simultaneously with bremsstrahlung, a small amount (several percent) of characteristic radiation is formed. Only 1-2% of the electrons hitting the anode cause bremsstrahlung, and the rest is a thermal effect. To concentrate electrons, the cathode has a guide cap. The part of the tungsten mirror on which the main flow of electrons falls is called the focus of the tube. The width of the radiation beam depends on its area (focus sharpness).
To power the tube, two sources are required: a high voltage source for the anode circuit and a low (6-8 V) source to power the incandescent circuit. Both sources must be independently regulated. By changing the anode voltage, the hardness of the X-ray radiation is regulated, and by changing the filament, the output circuit current and, accordingly, the radiation power are regulated.
The basic electrical diagram of a simple X-ray machine is shown in Fig. 4. The circuit has two transformers Tr.1 for high voltage and Tr.2 for incandescent power supply. The high voltage on the tube is regulated by autotransformer Tr.3, connected to the primary winding of transformer Tr.1. Switch K regulates the number of turns of the autotransformer winding. In this regard, the voltage of the secondary winding of the transformer, supplied to the anode of the tube, also changes, i.e. hardness is adjustable.
The filament current of the tube is regulated by a rheostat R connected to the circuit of the primary winding of transformer Tr.2. The anode circuit current is measured with a milliammeter. The voltage supplied to the electrodes of the tube is measured by a kilovoltmeter kV, or the voltage in the anode circuit can be judged by the position of switch K. The amount of filament current, regulated by a rheostat, is measured by ammeter A. In the circuit under consideration, the X-ray tube simultaneously rectifies a high alternating voltage.
It is easy to see that such a tube emits only one half-cycle of alternating current. Consequently, its power will be small. In order to increase the radiated power, many devices use high-voltage full-wave X-ray rectifiers. For this purpose, 4 special kenotrons are used, which are connected in a bridge circuit. An X-ray tube is included in one diagonal of the bridge.
3. Interaction of X-rays with matter
(coherent scattering, incoherent scattering, photoelectric effect).
When X-ray radiation falls on a body, it is reflected in a small amount from it, but mainly passes deep into it. In the mass of the body, radiation is partially absorbed, partially scattered, and partially passes through. Passing through the body, X-ray photons interact mainly with the electrons of atoms and molecules of the substance. The registration and use of X-ray radiation, as well as its impact on biological objects, is determined by the primary processes of interaction of the X-ray photon with electrons. Depending on the ratio of the photon energy E and the ionization energy A I, three main processes take place.
A) Coherent scattering.
Scattering of long-wave X-rays occurs essentially without changing the wavelength, and is called coherent. The interaction of a photon with the electrons of the inner shells, tightly bound to the nucleus, changes only its direction, without changing its energy, and therefore the wavelength (Fig. 5).
Coherent scattering occurs if the photon energy is less than the ionization energy: E = hn<А И. Так как энергия фотона и энергия атома не изменяется, то когерентное рассеяние не вызывает биологического действия. Однако при создании защиты от рентгеновского излучения следует учитывать возможность изменения направления первичного пучка.
b) Incoherent scattering (Compton effect).
In 1922, A. Compton, observing the scattering of hard X-rays, discovered a decrease in the penetrating power of the scattered beam compared to the incident beam. The scattering of X-rays with changes in wavelength is called the Compton effect. It occurs when a photon of any energy interacts with the electrons of the outer shells of atoms weakly bound to the nucleus (Fig. 6). An electron is removed from an atom (such electrons are called recoil electrons). The energy of the photon decreases (the wavelength increases accordingly), and the direction of its movement also changes. The Compton effect occurs if the energy of the X-ray photon is greater than the ionization energy: , . In this case, recoil electrons with kinetic energy E K appear. Atoms and molecules become ions. If E K is significant, then electrons can ionize neighboring atoms by collision, forming new (secondary) electrons.
V) Photo effect.
If the photon energy hn is sufficient to detach an electron, then when interacting with an atom, the photon is absorbed and the electron is separated from it. This phenomenon is called the photoelectric effect. The atom is ionized (photoionization). In this case, the electron acquires kinetic energy and, if the latter  is significant, it can ionize neighboring atoms by collision, forming new (secondary) electrons. If the photon energy is insufficient for ionization, then the photoelectric effect can manifest itself in the excitation of an atom or molecule. In some substances this leads to the subsequent emission of photons in the visible region (x-ray luminescence), and in tissues to the activation of molecules and photochemical reactions.
is significant, it can ionize neighboring atoms by collision, forming new (secondary) electrons. If the photon energy is insufficient for ionization, then the photoelectric effect can manifest itself in the excitation of an atom or molecule. In some substances this leads to the subsequent emission of photons in the visible region (x-ray luminescence), and in tissues to the activation of molecules and photochemical reactions.
The photoelectric effect is characteristic of photons with an energy of the order of 0.5-1 MeV.
The three main interaction processes discussed above are primary, they lead to subsequent secondary, tertiary, etc. phenomena. When X-rays enter a substance, a number of processes can occur before the energy of the X-ray photon is converted into the energy of thermal motion.
As a result of the above processes, the primary flux of X-ray radiation is weakened. This process obeys Bouguer's law. Let's write it in the form: Ф = Ф 0 e - mх, where m is the linear attenuation coefficient, depending on the nature of the substance (mainly on the density and atomic number) and on the wavelength of the radiation (photon energy). It can be represented as consisting of three terms corresponding to coherent scattering, incoherent scattering and photoelectric effect: ![]() .
.
Since the linear absorption coefficient depends on the density of the substance, they prefer to use the mass attenuation coefficient, which is equal to the ratio of the linear attenuation coefficient to the density of the absorber and does not depend on the density of the substance. The dependence of the X-ray flux (intensity) on the thickness of the absorbing filter is shown in Fig. 7 for H 2 O, Al, and Cu. Calculations show that a layer of water 36 mm thick, aluminum 15 mm and copper 1.6 mm reduce the intensity of X-ray radiation by 2 times. This thickness is called the half layer thickness d. If a substance attenuates x-ray radiation by half, then ![]() , Then
, Then ![]() , or ,
, or , ![]() ; ; . Knowing the thickness of the half layer, you can always determine m. Dimension.
; ; . Knowing the thickness of the half layer, you can always determine m. Dimension.
4. Use of X-rays in medicine
(fluoroscopy, radiography, X-ray tomography, fluorography, radiotherapy).
One of the most common uses of X-ray radiation in medicine is the examination of internal organs for diagnostic purposes - x-ray diagnostics.
For diagnostics, photons with an energy of 60-120 keV are used. In this case, the mass absorption coefficient is determined mainly by the photoelectric effect. Its value is proportional to l 3 (which manifests the high penetrating ability of hard radiation) and proportional to the third power of the number of atoms of the substance - absorber: , where K is the proportionality coefficient.
The human body consists of tissues and organs that have different absorption abilities with respect to x-ray radiation. Therefore, when it is illuminated with X-rays, a non-uniform shadow image is obtained on the screen, which gives a picture of the location of the internal organs and tissues. The densest radiation-absorbing tissues (heart, large vessels, bones) are visible dark, and the least absorbing tissues (lungs) are light.
In many cases, it is possible to judge their normal or pathological condition. X-ray diagnostics uses two main methods: fluoroscopy (transmission) and radiography (image). If the organ under study and the tissues surrounding it absorb the X-ray flux approximately equally, then special contrast agents are used. For example, on the eve of an X-ray examination of the stomach or intestines, a porridge-like mass of barium sulfate is given, in this case you can see their shadow image. In fluoroscopy and radiography, the x-ray image is a summary image of the entire thickness of the object through which the x-rays pass. Those details that are closest to the screen or film are most clearly outlined, while those that are distant become fuzzy and blurry. If there is a pathologically changed area in some organ, for example, destruction of lung tissue inside a large focus of inflammation, then in some cases this area may be “lost” on the radiograph in the sum of the shadows. To make it visible, a special method is used - tomography (layer-by-layer recording), which allows you to obtain images of individual layers of the studied area. This kind of layer-by-layer images-tomograms are obtained using a special apparatus called a tomograph, in which an X-ray tube (RT) and photographic film (FP) are periodically moved together, in antiphase, relative to the area of study. In this case, X-rays at any position of the RT will pass through the same point of the object (changed area), which is the center relative to which the periodic movement of the RT and FP occurs. A shadow image of the area will be captured on film. By changing the position of the “swing center”, it is possible to obtain layer-by-layer images of the object. Using a thin beam of X-ray radiation, a special screen (instead of FP) consisting of semiconductor detectors of ionizing radiation, it is possible to process the image during tomography using a computer. This modern version of tomography is called computed tomography. Tomography is widely used in the study of lungs, kidneys, gall bladder, stomach, bones, etc.
The brightness of the image on the screen and the exposure time on the film depend on the intensity of the x-ray radiation. When using it for diagnostics, the intensity cannot be high so as not to cause an undesirable biological effect. Therefore, there are a number of technical devices that improve image brightness at low X-ray intensities. One such device is an electron-optical converter.
Another example is fluorography, in which an image from a large X-ray luminescent screen is obtained on a sensitive small-format film. When shooting, a high-aperture lens is used, and the finished images are examined using a special magnifier.
Fluorography combines a greater ability to detect hidden diseases (diseases of the chest organs, gastrointestinal tract, paranasal sinuses, etc.) with significant throughput, and therefore is a very effective method of mass (in-line) research.
Since photographing an X-ray image during fluorography is done using photographic optics, the image on the fluorogram is reduced in comparison with the X-ray. In this regard, the resolution of a fluorogram (i.e., the discernibility of small details) is less than that of a conventional radiograph, however, it is greater than with fluoroscopy.
A device has been designed - a tomofluorograph, which makes it possible to obtain fluorograms of parts of the body and individual organs at a given depth - the so-called layer-by-layer images (slices) - tomofluorograms.
X-ray radiation is also used for therapeutic purposes (x-ray therapy). The biological effect of radiation is to disrupt the vital activity of cells, especially rapidly developing ones. In this regard, X-ray therapy is used to treat malignant tumors. It is possible to select a radiation dose sufficient to completely destroy the tumor with relatively minor damage to surrounding healthy tissue, which is restored due to subsequent regeneration.
Intensity- a quantitative characteristic of X-ray radiation, which is expressed by the number of rays emitted by the tube per unit time. The intensity of X-ray radiation is measured in milliamps. Comparing it with the intensity of visible light from a conventional incandescent lamp, we can draw an analogy: for example, a 20-watt lamp will shine with one intensity, or strength, and a 200-watt lamp will shine with another, while the quality of the light itself (its spectrum) is the same . The intensity of an X-ray is essentially the amount of it. Each electron creates one or more quanta of radiation at the anode, therefore, the number of X-rays when exposing an object is regulated by changing the number of electrons tending to the anode and the number of interactions of electrons with atoms of the tungsten target, which can be done in two ways:
1. By changing the degree of heating of the cathode spiral using a step-down transformer (the number of electrons generated during emission will depend on how hot the tungsten spiral is, and the number of radiation quanta will depend on the number of electrons);
2. By changing the value of the high voltage supplied by a step-up transformer to the poles of the tube - the cathode and the anode (the higher the voltage is applied to the poles of the tube, the more kinetic energy the electrons receive, which, due to their energy, can interact with several atoms of the anode substance in turn - see. rice. 5; electrons with low energy will be able to enter into fewer interactions).
The X-ray intensity (anode current) multiplied by the exposure time (tube operating time) corresponds to the X-ray exposure, which is measured in mAs (milliamperes per second). Exposure is a parameter that, like intensity, characterizes the number of rays emitted by the X-ray tube. The only difference is that the exposure also takes into account the operating time of the tube (for example, if the tube works for 0.01 seconds, then the number of rays will be one, and if 0.02 seconds, then the number of rays will be different - twice more). The radiation exposure is set by the radiologist on the control panel of the X-ray machine, depending on the type of examination, the size of the object being examined and the diagnostic task.
Rigidity- qualitative characteristics of x-ray radiation. It is measured by the magnitude of the high voltage on the tube - in kilovolts. Determines the penetrating power of x-rays. It is regulated by the high voltage supplied to the X-ray tube by a step-up transformer. The higher the potential difference is created across the electrodes of the tube, the more force the electrons are repelled from the cathode and rush to the anode and the stronger their collision with the anode. The stronger their collision, the shorter the wavelength of the resulting X-ray radiation and the higher the penetrating ability of this wave (or the hardness of the radiation, which, like the intensity, is regulated on the control panel by the voltage parameter on the tube - kilovoltage).
Rice. 7 - Dependence of wavelength on wave energy:
λ - wavelength;
E - wave energy
· The higher the kinetic energy of moving electrons, the stronger their impact on the anode and the shorter the wavelength of the resulting X-ray radiation. X-ray radiation with a long wavelength and low penetrating power is called “soft”; X-ray radiation with a short wavelength and high penetrating power is called “hard”.
 Rice. 8 - The relationship between the voltage on the X-ray tube and the wavelength of the resulting X-ray radiation:
Rice. 8 - The relationship between the voltage on the X-ray tube and the wavelength of the resulting X-ray radiation:
· The higher the voltage is applied to the poles of the tube, the stronger the potential difference appears across them, therefore, the kinetic energy of moving electrons will be higher. The voltage on the tube determines the speed of electrons and the force of their collision with the anode substance; therefore, the voltage determines the wavelength of the resulting X-ray radiation.
X-ray radiation (synonym X-rays) is with a wide range of wavelengths (from 8·10 -6 to 10 -12 cm). X-ray radiation occurs when charged particles, most often electrons, are decelerated in the electric field of atoms of a substance. The quanta formed in this case have different energies and form a continuous spectrum. The maximum energy of quanta in such a spectrum is equal to the energy of incident electrons. In (cm.) the maximum energy of X-ray quanta, expressed in kiloelectron-volts, is numerically equal to the magnitude of the voltage applied to the tube, expressed in kilovolts. When X-rays pass through a substance, they interact with the electrons of its atoms. For X-ray quanta with energies up to 100 keV, the most characteristic type of interaction is the photoelectric effect. As a result of such interaction, the energy of the quantum is completely spent on tearing the electron out of the atomic shell and imparting kinetic energy to it. As the energy of an X-ray quantum increases, the probability of the photoelectric effect decreases and the process of scattering of quantums by free electrons - the so-called Compton effect - becomes predominant. As a result of such interaction, a secondary electron is also formed and, in addition, a quantum is emitted with an energy lower than the energy of the primary quantum. If the energy of the X-ray quantum exceeds one megaelectron-volt, the so-called pairing effect can occur, in which an electron and a positron are formed (see). Consequently, when passing through a substance, the energy of X-ray radiation decreases, i.e., its intensity decreases. Since absorption of low-energy quanta occurs with a greater probability, the X-ray radiation is enriched with higher-energy quanta. This property of X-ray radiation is used to increase the average energy of quanta, i.e., to increase its hardness. An increase in the hardness of X-ray radiation is achieved using special filters (see). X-ray radiation is used for x-ray diagnostics (see) and (see). See also Ionizing radiation.
X-ray radiation (synonym: x-rays, x-rays) is quantum electromagnetic radiation with a wavelength from 250 to 0.025 A (or energy quanta from 5·10 -2 to 5·10 2 keV). In 1895 it was discovered by V.K. Roentgen. The spectral region of electromagnetic radiation adjacent to X-ray radiation, whose energy quanta exceed 500 keV, is called gamma radiation (see); radiation whose energy quanta are below 0.05 kev constitutes ultraviolet radiation (see).
Thus, representing a relatively small part of the vast spectrum of electromagnetic radiation, which includes both radio waves and visible light, X-ray radiation, like any electromagnetic radiation, propagates at the speed of light (in a vacuum of about 300 thousand km/sec) and is characterized by a wavelength λ ( the distance over which radiation travels in one oscillation period). X-ray radiation also has a number of other wave properties (refraction, interference, diffraction), but they are much more difficult to observe than longer wavelength radiation: visible light, radio waves.
X-ray spectra: a1 - continuous bremsstrahlung spectrum at 310 kV; a - continuous brake spectrum at 250 kV, a1 - spectrum filtered with 1 mm Cu, a2 - spectrum filtered with 2 mm Cu, b - K-series tungsten lines.
To generate X-ray radiation, X-ray tubes (see) are used, in which radiation occurs when fast electrons interact with atoms of the anode substance. There are two types of X-ray radiation: bremsstrahlung and characteristic. Bremsstrahlung X-rays have a continuous spectrum, similar to ordinary white light. The intensity distribution depending on the wavelength (Fig.) is represented by a curve with a maximum; towards long waves the curve falls flatly, and towards short waves it falls steeply and ends at a certain wavelength (λ0), called the short-wave boundary of the continuous spectrum. The value of λ0 is inversely proportional to the voltage on the tube. Bremsstrahlung occurs when fast electrons interact with atomic nuclei. The intensity of bremsstrahlung is directly proportional to the strength of the anode current, the square of the voltage across the tube and the atomic number (Z) of the anode substance.
If the energy of the electrons accelerated in the X-ray tube exceeds the value critical for the anode substance (this energy is determined by the voltage Vcr critical for this substance on the tube), then characteristic radiation occurs. The characteristic spectrum is lined; its spectral lines form series, designated by the letters K, L, M, N.
The K series is the shortest wavelength, the L series is longer wavelength, the M and N series are observed only in heavy elements (Vcr of tungsten for the K-series is 69.3 kV, for the L-series - 12.1 kV). Characteristic radiation arises as follows. Fast electrons knock atomic electrons out of their inner shells. The atom is excited and then returns to the ground state. In this case, electrons from the outer, less bound shells fill the spaces vacated in the inner shells, and photons of characteristic radiation are emitted with an energy equal to the difference between the energies of the atom in the excited and ground states. This difference (and therefore the photon energy) has a certain value characteristic of each element. This phenomenon underlies X-ray spectral analysis of elements. The figure shows the line spectrum of tungsten against the background of a continuous spectrum of bremsstrahlung.
The energy of electrons accelerated in the X-ray tube is converted almost entirely into thermal energy (the anode becomes very hot), only a small part (about 1% at a voltage close to 100 kV) is converted into bremsstrahlung energy.
The use of X-rays in medicine is based on the laws of absorption of X-rays by matter. The absorption of X-ray radiation is completely independent of the optical properties of the absorber substance. Colorless and transparent lead glass, used to protect personnel in x-ray rooms, almost completely absorbs x-rays. In contrast, a sheet of paper that is not transparent to light does not attenuate x-rays.
The intensity of a homogeneous (i.e., a certain wavelength) X-ray beam passing through an absorber layer decreases according to the exponential law (e-x), where e is the base of natural logarithms (2.718), and the exponent x is equal to the product of the mass attenuation coefficient (μ /p) cm 2 /g per thickness of the absorber in g/cm 2 (here p is the density of the substance in g/cm 3). The attenuation of X-ray radiation occurs due to both scattering and absorption. Accordingly, the mass attenuation coefficient is the sum of the mass absorption and scattering coefficients. The mass absorption coefficient increases sharply with increasing atomic number (Z) of the absorber (proportional to Z3 or Z5) and with increasing wavelength (proportional to λ3). This dependence on wavelength is observed within the absorption bands, at the boundaries of which the coefficient exhibits jumps.
The mass scattering coefficient increases with increasing atomic number of the substance. At λ≥0.3Å the scattering coefficient does not depend on the wavelength, at λ<0,ЗÅ он уменьшается с уменьшением λ.
A decrease in the absorption and scattering coefficients with decreasing wavelength causes an increase in the penetrating power of X-ray radiation. The mass absorption coefficient for bone [uptake is mainly due to Ca 3 (PO 4) 2 ] is almost 70 times greater than for soft tissue, where uptake is mainly due to water. This explains why the shadow of bones stands out so sharply against the background of soft tissue on radiographs.
The propagation of a non-uniform X-ray beam through any medium, along with a decrease in intensity, is accompanied by a change in the spectral composition and a change in the quality of the radiation: the long-wave part of the spectrum is absorbed to a greater extent than the short-wave part, the radiation becomes more uniform. Filtering out the long-wave part of the spectrum allows, during X-ray therapy of lesions located deep in the human body, to improve the ratio between deep and surface doses (see X-ray filters). To characterize the quality of an inhomogeneous beam of X-rays, the concept of “half-attenuation layer (L)” is used - a layer of substance that attenuates the radiation by half. The thickness of this layer depends on the voltage on the tube, the thickness and material of the filter. To measure half-attenuation layers, cellophane (up to 12 keV energy), aluminum (20-100 keV), copper (60-300 keV), lead and copper (>300 keV) are used. For X-rays generated at voltages of 80-120 kV, 1 mm of copper is equivalent in filtering capacity to 26 mm of aluminum, 1 mm of lead is equivalent to 50.9 mm of aluminum.
The absorption and scattering of X-ray radiation is due to its corpuscular properties; X-ray radiation interacts with atoms as a stream of corpuscles (particles) - photons, each of which has a certain energy (inversely proportional to the wavelength of X-ray radiation). The energy range of X-ray photons is 0.05-500 keV.
The absorption of X-ray radiation is due to the photoelectric effect: the absorption of a photon by the electron shell is accompanied by the ejection of an electron. The atom is excited and, returning to the ground state, emits characteristic radiation. The emitted photoelectron carries away all the energy of the photon (minus the binding energy of the electron in the atom).
X-ray scattering is caused by electrons in the scattering medium. A distinction is made between classical scattering (the wavelength of the radiation does not change, but the direction of propagation changes) and scattering with a change in wavelength - the Compton effect (the wavelength of the scattered radiation is greater than that of the incident radiation). In the latter case, the photon behaves like a moving ball, and the scattering of photons occurs, according to Comton’s figurative expression, like playing billiards with photons and electrons: colliding with an electron, the photon transfers part of its energy to it and is scattered, having less energy (accordingly, the wavelength of the scattered radiation increases), an electron flies out of the atom with recoil energy (these electrons are called Compton electrons, or recoil electrons). Absorption of X-ray energy occurs during the formation of secondary electrons (Compton and photoelectrons) and the transfer of energy to them. The energy of X-ray radiation transferred to a unit mass of a substance determines the absorbed dose of X-ray radiation. The unit of this dose 1 rad corresponds to 100 erg/g. Due to the absorbed energy, a number of secondary processes occur in the absorber substance, which are important for X-ray dosimetry, since it is on them that the methods for measuring X-ray radiation are based. (see Dosimetry).
All gases and many liquids, semiconductors and dielectrics increase electrical conductivity when exposed to X-rays. Conductivity is detected by the best insulating materials: paraffin, mica, rubber, amber. The change in conductivity is caused by ionization of the medium, i.e., the separation of neutral molecules into positive and negative ions (ionization is produced by secondary electrons). Ionization in air is used to determine X-ray exposure dose (dose in air), which is measured in roentgens (see Ionizing Radiation Doses). At a dose of 1 r, the absorbed dose in air is 0.88 rad.
Under the influence of X-ray radiation, as a result of the excitation of molecules of a substance (and during the recombination of ions), in many cases a visible glow of the substance is excited. At high intensities of X-ray radiation, a visible glow is observed in air, paper, paraffin, etc. (with the exception of metals). The highest yield of visible luminescence is provided by crystalline phosphors such as Zn·CdS·Ag-phosphorus and others used for fluoroscopy screens.
Under the influence of x-ray radiation, various chemical processes can also occur in a substance: decomposition of silver halide compounds (a photographic effect used in x-ray photography), decomposition of water and aqueous solutions of hydrogen peroxide, changes in the properties of celluloid (turbidity and release of camphor), paraffin (turbidity and bleaching) .
As a result of complete conversion, all the energy absorbed by the chemically inert substance, the x-ray radiation, is converted into heat. Measuring very small amounts of heat requires highly sensitive methods, but is the main method for absolute measurements of X-ray radiation.
Secondary biological effects from exposure to x-ray radiation are the basis of medical x-ray therapy (see). X-ray radiation, whose quanta are 6-16 keV (effective wavelengths from 2 to 5 Å), is almost completely absorbed by the skin tissue of the human body; these are called boundary rays, or sometimes Bucca's rays (see Bucca's rays). For deep X-ray therapy, hard filtered radiation with effective energy quanta from 100 to 300 keV is used.
The biological effect of X-ray radiation should be taken into account not only during X-ray therapy, but also during X-ray diagnostics, as well as in all other cases of contact with X-ray radiation that require the use of radiation protection (see).
X-RAY
X-RAY
invisible radiation capable of penetrating, although to varying degrees, all substances. It is electromagnetic radiation with a wavelength of about 10-8 cm. Like visible light, X-ray radiation causes blackening of photographic film. This property is important for medicine, industry and scientific research. Passing through the object under study and then falling onto the photographic film, X-ray radiation depicts its internal structure on it. Since the penetrating power of X-ray radiation varies for different materials, parts of the object that are less transparent to it produce lighter areas in the photograph than those through which the radiation penetrates well. Thus, bone tissue is less transparent to x-rays than the tissue that makes up the skin and internal organs. Therefore, on an x-ray, the bones will appear as lighter areas and the fracture site, which is more transparent to radiation, can be detected quite easily. X-rays are also used in dentistry to detect caries and abscesses in the roots of teeth, and in industry to detect cracks in castings, plastics and rubbers. X-rays are used in chemistry to analyze compounds and in physics to study the structure of crystals. An X-ray beam passing through a chemical compound produces characteristic secondary radiation, the spectroscopic analysis of which allows the chemist to determine the composition of the compound. When falling on a crystalline substance, a beam of X-rays is scattered by the atoms of the crystal, giving a clear, regular picture of spots and stripes on a photographic plate, which makes it possible to establish the internal structure of the crystal. The use of X-rays in cancer treatment is based on the fact that it kills cancer cells. However, it can also have undesirable effects on normal cells. Therefore, extreme caution must be exercised when using X-rays in this manner. X-ray radiation was discovered by the German physicist W. Roentgen (1845-1923). His name is immortalized in several other physical terms associated with this radiation: the roentgen is the international unit of dose of ionizing radiation; a picture taken in an X-ray machine is called a radiograph; The field of radiological medicine that uses x-rays to diagnose and treat diseases is called radiology. Roentgen discovered radiation in 1895 while professor of physics at the University of Würzburg. While conducting experiments with cathode rays (electron flows in discharge tubes), he noticed that a screen located near a vacuum tube, covered with crystalline barium cyanoplatinite, glowed brightly, although the tube itself was covered with black cardboard. Roentgen further established that the penetrating ability of the unknown rays he discovered, which he called X-rays, depended on the composition of the absorbing material. He also obtained an image of the bones of his own hand by placing it between a discharge tube with cathode rays and a screen coated with barium cyanoplatinite. Roentgen's discovery was followed by experiments by other researchers who discovered many new properties and applications of this radiation. A major contribution was made by M. Laue, W. Friedrich and P. Knipping, who demonstrated in 1912 the diffraction of X-ray radiation when passing through a crystal; W. Coolidge, who in 1913 invented a high-vacuum X-ray tube with a heated cathode; G. Moseley, who established in 1913 the relationship between the wavelength of radiation and the atomic number of an element; G. and L. Bragg, who received the Nobel Prize in 1915 for developing the fundamentals of X-ray structural analysis. RECEIVING X-RAYS X-ray radiation occurs when electrons moving at high speeds interact with matter. When electrons collide with atoms of any substance, they quickly lose their kinetic energy. In this case, most of it turns into heat, and a small fraction, usually less than 1%, is converted into X-ray energy. This energy is released in the form of quanta - particles called photons, which have energy but whose rest mass is zero. X-ray photons differ in their energy, which is inversely proportional to their wavelength. The conventional method of producing X-rays produces a wide range of wavelengths, which is called the X-ray spectrum. The spectrum contains pronounced components, as shown in Fig. 1. The broad “continuum” is called the continuous spectrum or white radiation. The sharp peaks superimposed on it are called characteristic X-ray emission lines. Although the entire spectrum is the result of collisions of electrons with matter, the mechanisms for the appearance of its wide part and lines are different. A substance consists of a large number of atoms, each of which has a nucleus surrounded by electron shells, and each electron in the shell of an atom of a given element occupies a certain discrete energy level. Typically these shells, or energy levels, are designated by the symbols K, L, M, etc., starting from the shell closest to the nucleus. When an incident electron with sufficiently high energy collides with one of the electrons associated with the atom, it knocks that electron out of its shell. The empty space is occupied by another electron from the shell, which corresponds to a higher energy. This latter gives up excess energy by emitting an X-ray photon. Since shell electrons have discrete energy values, the resulting X-ray photons also have a discrete spectrum. This corresponds to sharp peaks for certain wavelengths, the specific values of which depend on the target element. The characteristic lines form the K-, L- and M-series, depending on which shell (K, L or M) the electron was removed from. The relationship between X-ray wavelength and atomic number is called Moseley's law (Figure 2).
Rice. 1. A CONVENTIONAL X-RAY SPECTRUM consists of a continuous spectrum (continuum) and characteristic lines (sharp peaks). The K/ia and K/ib lines arise due to interactions of accelerated electrons with electrons of the inner K-shell.
 Rice. 2. THE WAVELENGTH OF CHARACTERISTIC X-RAY RADIATION emitted by chemical elements depends on the atomic number of the element. The curve follows Moseley's law: the higher the atomic number of the element, the shorter the wavelength of the characteristic line.
Rice. 2. THE WAVELENGTH OF CHARACTERISTIC X-RAY RADIATION emitted by chemical elements depends on the atomic number of the element. The curve follows Moseley's law: the higher the atomic number of the element, the shorter the wavelength of the characteristic line.
If an electron collides with a relatively heavy nucleus, it is decelerated, and its kinetic energy is released in the form of an X-ray photon of approximately the same energy. If it flies past the nucleus, it will lose only part of its energy, and the rest will be transferred to other atoms that come across its path. Each act of energy loss leads to the emission of a photon with some energy. A continuous X-ray spectrum appears, the upper limit of which corresponds to the energy of the fastest electron. This is the mechanism for the formation of a continuous spectrum, and the maximum energy (or minimum wavelength) that fixes the boundary of the continuous spectrum is proportional to the accelerating voltage, which determines the speed of the incident electrons. Spectral lines characterize the material of the bombarded target, and the continuous spectrum is determined by the energy of the electron beam and is practically independent of the target material. X-ray radiation can be obtained not only by electron bombardment, but also by irradiating a target with X-ray radiation from another source. In this case, however, most of the energy of the incident beam goes into the characteristic X-ray spectrum and a very small proportion of it falls into the continuous one. It is obvious that the beam of incident X-ray radiation must contain photons whose energy is sufficient to excite the characteristic lines of the bombarded element. The high percentage of energy per characteristic spectrum makes this method of excitation of X-ray radiation convenient for scientific research. X-ray tubes. To produce X-rays through the interaction of electrons with matter, you need to have a source of electrons, a means of accelerating them to high speeds, and a target that can withstand electron bombardment and produce X-rays of the required intensity. The device that contains all this is called an X-ray tube. Early researchers used "deeply evacuated" tubes such as modern gas-discharge tubes. The vacuum in them was not very high. Discharge tubes contain small amounts of gas, and when a large potential difference is applied to the tube's electrodes, the gas atoms are converted into positive and negative ions. The positive ones move towards the negative electrode (cathode) and, falling on it, knock out electrons from it, and they, in turn, move towards the positive electrode (anode) and, bombarding it, create a stream of X-ray photons. In the modern X-ray tube developed by Coolidge (Fig. 3), the source of electrons is a tungsten cathode heated to a high temperature. Electrons are accelerated to high speeds by the high potential difference between the anode (or anti-cathode) and the cathode. Since the electrons must reach the anode without colliding with atoms, a very high vacuum is necessary, which requires the tube to be well evacuated. This also reduces the probability of ionization of the remaining gas atoms and the resulting side currents.
 Rice. 3. COOLIDGE X-RAY TUBE. When bombarded by electrons, the tungsten anticathode emits characteristic X-ray radiation. The cross section of the X-ray beam is smaller than the actual irradiated area. 1 - electron beam; 2 - cathode with a focusing electrode; 3 - glass shell (tube); 4 - tungsten target (anti-cathode); 5 - cathode filament; 6 - actual irradiated area; 7 - effective focal spot; 8 - copper anode; 9 - window; 10 - scattered x-ray radiation.
Rice. 3. COOLIDGE X-RAY TUBE. When bombarded by electrons, the tungsten anticathode emits characteristic X-ray radiation. The cross section of the X-ray beam is smaller than the actual irradiated area. 1 - electron beam; 2 - cathode with a focusing electrode; 3 - glass shell (tube); 4 - tungsten target (anti-cathode); 5 - cathode filament; 6 - actual irradiated area; 7 - effective focal spot; 8 - copper anode; 9 - window; 10 - scattered x-ray radiation.
The electrons are focused onto the anode by a specially shaped electrode surrounding the cathode. This electrode is called a focusing electrode and, together with the cathode, forms the “electronic spotlight” of the tube. The anode subjected to electron bombardment must be made of a refractory material, since most of the kinetic energy of the bombarding electrons is converted into heat. In addition, it is desirable that the anode be made of a material with a high atomic number, because The X-ray yield increases with increasing atomic number. The anode material most often chosen is tungsten, whose atomic number is 74. The design of X-ray tubes can vary depending on the conditions of use and the requirements. X-RAY DETECTION All methods for detecting X-rays are based on their interaction with matter. Detectors can be of two types: those that provide an image and those that do not. The first include X-ray fluorography and fluoroscopy devices, in which a beam of X-ray radiation passes through the object under study, and the transmitted radiation hits a luminescent screen or photographic film. The image appears due to the fact that different parts of the object under study absorb radiation differently - depending on the thickness of the substance and its composition. In detectors with a fluorescent screen, the X-ray energy is converted into a directly observable image, while in radiography it is recorded on a sensitive emulsion and can only be observed after the film has been developed. The second type of detectors includes a wide variety of devices in which the energy of X-ray radiation is converted into electrical signals that characterize the relative intensity of the radiation. These include ionization chambers, Geiger counters, proportional counters, scintillation counters, and some specialty cadmium sulfide and selenide detectors. Currently, the most effective detectors can be considered scintillation counters, which work well over a wide energy range. see also PARTICLE DETECTORS. The detector is selected taking into account the conditions of the task. For example, if you need to accurately measure the intensity of diffracted X-ray radiation, then counters are used that allow you to make measurements with an accuracy of a fraction of a percent. If you need to register a lot of diffracted beams, then it is advisable to use X-ray film, although in this case it is impossible to determine the intensity with the same accuracy. X-RAY AND GAMMA DEFECTOSCOPY One of the most common uses of X-rays in industry is in materials quality control and flaw detection. The X-ray method is non-destructive, so that the material being tested, if found to satisfy the necessary requirements, can then be used for its intended purpose. Both X-ray and gamma flaw detection are based on the penetrating ability of X-ray radiation and the characteristics of its absorption in materials. The penetrating power is determined by the energy of the X-ray photons, which depends on the accelerating voltage in the X-ray tube. Therefore, thick samples and samples made of heavy metals, such as gold and uranium, require an X-ray source with a higher voltage to study them, while for thin samples a source with a lower voltage is sufficient. For gamma flaw detection of very large castings and large rolled products, betatrons and linear accelerators are used, accelerating particles to energies of 25 MeV or more. The absorption of X-ray radiation in a material depends on the thickness of the absorber d and the absorption coefficient m and is determined by the formula I = I0e-md, where I is the intensity of the radiation passing through the absorber, I0 is the intensity of the incident radiation, and e = 2.718 is the base of natural logarithms. For a given material at a given wavelength (or energy) of x-ray radiation, the absorption coefficient is a constant. But the radiation of an X-ray source is not monochromatic, but contains a wide spectrum of wavelengths, as a result of which absorption at the same thickness of the absorber depends on the wavelength (frequency) of the radiation. X-ray radiation is widely used in all industries related to metal forming. It is also used for testing artillery barrels, food products, plastics, and for testing complex devices and systems in electronic technology. (Neutronography, which uses neutron beams instead of X-rays, is used for similar purposes.) X-rays are also used for other purposes, such as examining paintings to determine their authenticity or detecting additional layers of paint on top of the base layer. X-RAY DIFFRACTION X-ray diffraction provides important information about solids—their atomic structure and crystal shape—as well as about liquids, amorphous solids, and large molecules. The diffraction method is also used to accurately (with an error of less than 10-5) determine interatomic distances, identify stresses and defects, and determine the orientation of single crystals. Using the diffraction pattern, you can identify unknown materials, as well as detect the presence of impurities in the sample and identify them. The importance of the X-ray diffraction method for the progress of modern physics can hardly be overestimated, since modern understanding of the properties of matter is ultimately based on data on the arrangement of atoms in various chemical compounds, the nature of the bonds between them and structural defects. The main tool for obtaining this information is the X-ray diffraction method. X-ray diffraction crystallography is critical for determining the structures of complex large molecules, such as deoxyribonucleic acid (DNA) molecules, the genetic material of living organisms. Immediately after the discovery of X-rays, scientific and medical interest focused both on the ability of this radiation to penetrate bodies and on its nature. Experiments on diffraction of X-ray radiation by slits and diffraction gratings showed that it belongs to electromagnetic radiation and has a wavelength of the order of 10-8-10-9 cm. Even earlier, scientists, in particular W. Barlow, guessed that the regular and symmetrical shape of natural crystals is due to the ordered arrangement of atoms that form the crystal. In some cases, Barlow was able to correctly predict the crystal structure. The value of the predicted interatomic distances was 10-8 cm. The fact that the interatomic distances turned out to be on the order of the X-ray wavelength made it possible, in principle, to observe their diffraction. The result was the design of one of the most important experiments in the history of physics. M. Laue organized an experimental test of this idea, which was carried out by his colleagues W. Friedrich and P. Knipping. In 1912, the three of them published their work on the results of X-ray diffraction. Principles of X-ray diffraction. To understand the phenomenon of X-ray diffraction, we need to consider in order: first, the spectrum of X-ray radiation, second, the nature of the crystal structure, and third, the phenomenon of diffraction itself. As mentioned above, characteristic X-ray radiation consists of a series of spectral lines with a high degree of monochromaticity, determined by the anode material. Using filters you can highlight the most intense ones. Therefore, by choosing the anode material appropriately, it is possible to obtain a source of almost monochromatic radiation with a very precisely defined wavelength. Characteristic radiation wavelengths typically range from 2.285 for chromium to 0.558 for silver (the values for the various elements are known to six significant figures). The characteristic spectrum is superimposed on a continuous “white” spectrum of much lower intensity, due to the deceleration of incident electrons in the anode. Thus, two types of radiation can be obtained from each anode: characteristic and bremsstrahlung, each of which plays an important role in its own way. Atoms in a crystal structure are arranged with regular periodicity, forming a sequence of identical cells - a spatial lattice. Some lattices (such as those for most common metals) are quite simple, while others (such as those for protein molecules) are quite complex. The following is characteristic of a crystal structure: if one moves from a certain given point of one cell to the corresponding point of an adjacent cell, then exactly the same atomic environment will be revealed. And if a certain atom is located at one point or another in one cell, then the same atom will be located at an equivalent point in any neighboring cell. This principle is strictly valid for a perfect, ideally ordered crystal. However, many crystals (for example, metal solid solutions) are disordered to one degree or another, i.e. crystallographically equivalent sites can be occupied by different atoms. In these cases, it is not the position of each atom that is determined, but only the position of the atom “statistically averaged” over a large number of particles (or cells). The phenomenon of diffraction is discussed in the article OPTICS and the reader may refer to that article before proceeding further. It shows that if waves (for example, sound, light, x-rays) pass through a small slit or hole, then the latter can be considered as a secondary source of waves, and the image of the slit or hole consists of alternating light and dark stripes. Further, if there is a periodic structure of holes or slits, then as a result of the amplifying and weakening interference of rays coming from different holes, a clear diffraction pattern appears. X-ray diffraction is a collective scattering phenomenon in which the role of holes and scattering centers is played by periodically arranged atoms of the crystal structure. The mutual enhancement of their images at certain angles produces a diffraction pattern similar to that which would arise when light was diffraction on a three-dimensional diffraction grating. Scattering occurs due to the interaction of incident X-rays with electrons in the crystal. Due to the fact that the wavelength of X-rays is of the same order of magnitude as the size of the atom, the wavelength of the scattered X-rays is the same as the incident X-rays. This process is the result of forced oscillations of electrons under the influence of incident X-rays. Consider now an atom with a cloud of bound electrons (surrounding the nucleus) that is hit by X-rays. Electrons in all directions simultaneously scatter the incident radiation and emit their own X-ray radiation of the same wavelength, although of different intensity. The intensity of the scattered radiation is related to the atomic number of the element, because atomic number is equal to the number of orbital electrons that can participate in scattering. (This dependence of the intensity on the atomic number of the scattering element and on the direction in which the intensity is measured is characterized by the atomic scattering factor, which plays an extremely important role in the analysis of the structure of crystals.) Let us select in the crystal structure a linear chain of atoms located at the same distance from each other, and consider their diffraction pattern. It has already been noted that the X-ray spectrum consists of a continuous part ("continuum") and a set of more intense lines characteristic of the element that is the anode material. Let's say we filtered the continuous spectrum and got a nearly monochromatic beam of X-rays directed at our linear chain of atoms. The condition of amplification (amplifying interference) is satisfied if the difference in the paths of the waves scattered by neighboring atoms is a multiple of the wavelength. If the beam is incident at an angle a0 to a line of atoms separated by intervals a (period), then for the diffraction angle a the path difference corresponding to the amplification will be written as a(cos a - cosa0) = hl, where l is the wavelength and h integer (Fig. 4 and 5).
 Rice. 4. Amplification of an X-ray beam occurs when the difference in the path of waves scattered by neighboring atoms is equal to an integer multiple of the wavelength. Here a0 is the angle of incidence, a is the diffraction angle, a is the distance between the atoms.
Rice. 4. Amplification of an X-ray beam occurs when the difference in the path of waves scattered by neighboring atoms is equal to an integer multiple of the wavelength. Here a0 is the angle of incidence, a is the diffraction angle, a is the distance between the atoms.
 Rice. 5. SOLUTION OF THE LAUE EQUATIONS for each value of h can be represented as a family of cones, the common axis of which is directed along the crystallographic axis (similar pictures can be drawn for the other two axes). An effective method for studying crystal structures is based on the Laue equations.
Rice. 5. SOLUTION OF THE LAUE EQUATIONS for each value of h can be represented as a family of cones, the common axis of which is directed along the crystallographic axis (similar pictures can be drawn for the other two axes). An effective method for studying crystal structures is based on the Laue equations.
To extend this approach to a three-dimensional crystal, it is only necessary to select rows of atoms along two other directions in the crystal and solve the three equations thus obtained jointly for the three crystal axes with periods a, b and c. The other two equations have the form
 <=""
div="" style="border-style: none;">These are the three fundamental Laue equations for X-ray diffraction, with the numbers h, k and c being the Miller indices for the diffraction plane. see also CRYSTALS AND CRYSTALLOGRAPHY. Considering any of the Laue equations, for example the first, you can notice that since a, a0, l are constants, and h = 0, 1, 2, ..., its solution can be represented as a set of cones with a common axis a (Fig. . 5). The same is true for directions b and c. In the general case of three-dimensional scattering (diffraction), the three Laue equations must have a common solution, i.e. three diffraction cones located on each of the axes must intersect; the general line of intersection is shown in Fig. 6. The joint solution of the equations leads to the Bragg-Wolfe law:
<=""
div="" style="border-style: none;">These are the three fundamental Laue equations for X-ray diffraction, with the numbers h, k and c being the Miller indices for the diffraction plane. see also CRYSTALS AND CRYSTALLOGRAPHY. Considering any of the Laue equations, for example the first, you can notice that since a, a0, l are constants, and h = 0, 1, 2, ..., its solution can be represented as a set of cones with a common axis a (Fig. . 5). The same is true for directions b and c. In the general case of three-dimensional scattering (diffraction), the three Laue equations must have a common solution, i.e. three diffraction cones located on each of the axes must intersect; the general line of intersection is shown in Fig. 6. The joint solution of the equations leads to the Bragg-Wolfe law:
 Rice. 6. THE GENERAL SOLUTION OF THE LAUE EQUATIONS corresponds to the intersection of three cones with axes a, b, c, having a common straight line R.
Rice. 6. THE GENERAL SOLUTION OF THE LAUE EQUATIONS corresponds to the intersection of three cones with axes a, b, c, having a common straight line R.
l = 2(d/n)sinq, where d is the distance between the planes with indices h, k and c (period), n = 1, 2, ... are integers (diffraction order), and q is the angle formed an incident beam (as well as a diffracting one) with the crystal plane in which diffraction occurs. Analyzing the Bragg-Wolfe law equation for a single crystal located in the path of a monochromatic X-ray beam, we can conclude that diffraction is not easy to observe, because the quantities l and q are fixed, and sinq< 1. При таких условиях, чтобы имела место дифракция для рентгеновского излучения с длиной волны l, плоскость кристалла с периодом d должна быть повернута на правильный угол q. Для того чтобы реализовать это маловероятное событие, применяются различные методики. METHODS OF DIFFRACTION ANALYSIS Laue method. The Laue method uses a continuous "white" spectrum of X-ray radiation, which is directed at a stationary single crystal. For a specific value of period d, the wavelength corresponding to the Bragg-Wulf condition is automatically selected from the entire spectrum. The Lauegrams obtained in this way make it possible to judge the directions of the diffracted beams and, consequently, the orientations of the planes of the crystal, which also makes it possible to draw important conclusions regarding the symmetry, orientation of the crystal and the presence of defects in it. In this case, however, information about the spatial period d is lost. In Fig. 7 shows an example of a Lauegram. The X-ray film was located on the side of the crystal opposite to that on which the X-ray beam from the source fell.
 Rice. 7. LAUEGRAM. X-rays of a wide spectral range are passed through a stationary crystal. Diffraction beams correspond to spots on the Lauegram.
Rice. 7. LAUEGRAM. X-rays of a wide spectral range are passed through a stationary crystal. Diffraction beams correspond to spots on the Lauegram.
Debye-Scherrer method (for polycrystalline samples). Unlike the previous method, monochromatic radiation is used here (l = const), and the angle q is varied. This is achieved by using a polycrystalline sample consisting of numerous small crystallites of random orientation, among which there are some that satisfy the Bragg-Wulf condition. Diffracted beams form cones, the axis of which is directed along the X-ray beam. For imaging, a narrow strip of X-ray film in a cylindrical cassette is usually used, and the X-rays are distributed along the diameter through holes in the film. The Debyegram obtained in this way (Fig. 8) contains accurate information about period d, i.e. about the structure of the crystal, but does not provide the information that the Lauegram contains. Therefore, both methods complement each other. Let's consider some applications of the Debye-Scherrer method.
The effect of X-ray radiation on matter is determined by the primary processes of interaction of the X-ray photon with the electrons of atoms and molecules of the substance.
3. X-ray computed tomography.
The X-ray computed tomography method is based on reconstructing an image of a certain section (slice) of the patient’s body by recording a large number of X-ray projections of this section, performed at different angles (Fig. 5). Information from sensors that record these projections enters a computer, which, using a special program, calculates distribution sample density in the section under study and displays it on the display screen. The cross-sectional image of the patient’s body obtained in this way is characterized by excellent clarity and high information content. The program allows, if necessary, increase image contrast tens and even hundreds of times. This expands the diagnostic capabilities of the method.
Rice. 5. Scheme of x-ray examination of a section of the organ under study (point 1 and point 2 - two consecutive positions of the x-ray source)
4. With fluorography The image from the large screen is recorded on sensitive small-format film (Fig. 6). During analysis, images are examined using a special magnifier.
This method is used for mass population surveys. In this case, the radiation exposure to the patient is much less than in traditional fluoroscopy.
X-ray therapy- use of X-ray radiation to destroy malignant tumors.
The biological effect of radiation is to disrupt the vital activity of rapidly multiplying tumor cells. In this case, the energy of R - photons is 150-200 keV.
Visiographs (devices with digital X-ray image processing) in modern dentistry
In dentistry, X-ray examination is the main diagnostic method. However, a number of traditional organizational and technical features of x-ray diagnostics make it not entirely comfortable for both the patient and dental clinics. This is, first of all, the need for patient contact with ionizing radiation, which often creates a significant radiation load on the body; it is also the need for a photoprocess, and therefore the need for photoreagents, including toxic ones. This is, finally, a bulky archive, heavy folders and envelopes with x-ray films.
In addition, the current level of development of dentistry makes subjective assessment of radiographs by the human eye insufficient. As it turned out, out of the variety of shades of gray contained in an x-ray image, the eye perceives only 64.
Obviously, to obtain a clear and detailed image of the hard tissues of the dental-facial system with minimal radiation exposure, other solutions are needed. Today, the search has led to the creation of so-called radiographic systems, videographs - digital radiography systems (1987, Trophy company).
Without technical details, the operating principle of such systems is as follows. X-ray radiation passes through the object not to a photosensitive film, but to a special intraoral sensor (a special electronic matrix). The corresponding signal from the matrix is transmitted to a digitizing device (analog-to-digital converter, ADC) connected to the computer, which converts it into digital form. Special software creates an X-ray image on a computer screen and allows you to process it, save it on a hard or flexible storage medium (hard drive, disk), and print it as a file as a picture.
In a digital system, an X-ray image is a collection of points that correspond to different shades of gray. The optimization of information display provided by the program makes it possible to obtain a frame that is optimal in brightness and contrast with a relatively low radiation dose.
In modern systems, created, for example, by Trophy (France) or Schick (USA), 4096 shades of gray are used when forming a frame, the exposure time depends on the object of study and, on average, is hundredths - tenths of a second, reducing radiation exposure in relation to to film - up to 90% for intraoral systems, up to 70% for panoramic videographers.
When processing images, videographers can:
1. Receive positive and negative images, pseudo-color images, relief images.
2. Increase contrast and enlarge the image fragment of interest.
3. Assess changes in the density of dental tissues and bone structures, control the uniformity of filling the canals.
4. In endodontics, determine the length of a canal of any curvature, and in surgery, select the size of the implant with an accuracy of 0.1 mm.
The unique Caries detector system with elements of artificial intelligence when analyzing an image allows you to detect caries in the spot stage, root caries and hidden caries.
Solve problems:
1. How many times is the maximum energy of an X-ray bremsstrahlung quantum produced at a tube voltage of 80 kV greater than the energy of a photon corresponding to green light with a wavelength of 500 nm?
2. Determine the minimum wavelength in the spectrum of radiation resulting from the deceleration of electrons accelerated in the betatron to an energy of 60 MeV on the target.
3. The half-attenuation layer of monochromatic X-rays in a certain substance is 10 mm. Find the attenuation rate of this radiation in this substance.
[*] Φ l is the ratio of energy emitted in a narrow range of wavelengths in 1 s. to the width of this interval
* “F” in formula (4) refers to the entire range of emitted wavelengths and is often called “Integral energy flux”.