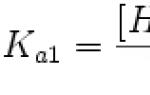Determination of acidity constants of weak acids. Acidity and basicity in water Index of the acidity constant of lactic acid
Self-ionization of water
Water, even after repeated distillation, retains the ability to conduct electric current. This ability of water is due to its self-ionization.
$2H_2O ↔ H_3O^+ + OH^-$
The thermodynamic equilibrium constant has the form:
Picture 1.
where $a_X^(rel)=\frac(a_X^(equal))(a_X^0)$ is the relative activity of the $X$ particle in the equilibrium system;
$aX^(equal)$ is the absolute activity of particle $X$ in an equilibrium system;
$(a_x)^0$ is the absolute activity of $X$ in the thermodynamic state of the system.
The relative activity of water at equilibrium is practically equal to unity, since the degree of reaction is very small (if we take theoretically non-ionized water as the standard state.
The activity coefficients of $OH^-$ and $H_3O^+$ ions will be close to unity in clean water. The equilibrium of the reaction is strongly shifted to the left. The relative activities of $OH^-$ and $H_3O^+$ are almost equal to their molar concentrations. Where
$(K_a)^0 \sim K_(auto) = $
where $ and $ are molar concentrations;
$K_(auto)$ - water autoprolysis constant equal to $1.00\cdot 10^(-14) \mol^2/l^2$ at $25^\circ \C.$
In pure water the concentrations of $ and $ will be equal, therefore
$==\sqrt(10^(-14))=10^(-7)$ at $25^\circ \ C.$
For ease of calculation, the concentration is indicated as a negative logarithm, denoted as $pH$:
$pH= -lg $
Indicators $pH$ for pure water are $7$, in acidic solutions $pH$ 7$.
Acid dissociation and acidity constant
For acid $AH$, dissociation can be expressed by the equation:
$AH + H_2O ↔ A^- + H_3O^+$
In a state of equilibrium, the relative density of water changes slightly when moving from one acid to another, and with infinite dilution it approaches zero. Therefore, the thermodynamic acidity constant $K_a^0$ ($AH$) is used.
The ratio of activity coefficients is the same for all acids and is equal to unity if the processes occur in dilute solutions.
Then, in a dilute aqueous solution, the acidity constant $Ka (AH$) is used as a measure of acid strength, which can be determined by the formula:
$Ka (AH)=\frac()()$
The formula displays the molar concentration of particles at a fixed temperature $(25^\circ \C)$ in a state of equilibrium.
The higher the acidity constant, the higher the degree of dissociation, the stronger the acid. For calculations and characterization of acidity, the negative logarithm of the acidity constant $pKa$ is used.
$pKa (AH)= -lgKa (AH)$
The higher the value of the acidity constant, the weaker the acid.
The value of the acidity constant is equal to the $pH$ value of the solution at which the acid will be half ionized:
$pKa (AH) = pH - log \frac()()$
The value characterizing the acidity of water molecules in an aqueous solution is equal to:
$Ka=\frac()()=\frac(Ka_(auto))()=\frac(10^(-14))(55.5)$
Thus, at a temperature of $25^\circ C$, $pKa (H_2O) = 15.7$. This value characterizes the acidity of water molecules in solution.
For hydronium ion $pKa (H_3O^+) = pK_(auto) - pKa = 14-15.7 = -1.7.$
The $pKa$ values are tabular data. However, for acids with $pKa 0$ the table data will be inaccurate.
It is possible to determine acidity constants in water by direct measurement of the concentrations of $A^-$ and $AH$ only when acid dissociation occurs to at least some extent, even barely noticeable.
If the acid is very weak, such that it practically does not dissociate, then the concentration of $A^-$ cannot be accurately measured. If, on the contrary, the acid is so strong that it dissociates almost completely, then it is impossible to measure the concentration of $AH$. In this case, indirect methods for determining acidity will be used.
Base ionization constant
To express the dissociation constant of a base in water, we use the equation:
$B + H_2O ↔ BH^+ + OH^-$
The basicity constant is:
$Kb=\frac())([B])$
Recently, basicity constants are practically not used in calculations, since from the acidity constant of the conjugate acid one can obtain all the necessary information about the base $BH^+.$
$BH^+ + H_2O ↔ B + H_3O^+$
$Ka (BH^+) = \frac([B])()$
The acidity constant of an acid will be a measure of strength:
- $AH$ or $BH^+$ as proton donors;
- $A^-$ or $B$ as proton acceptors;
- a strong acid $AH$ or $BH^+$ corresponds to a weak conjugate base $A^-$ or $B$, and then the value of $pKa$ is small or negative;
- a strong base $A^-$ or $B$ corresponds to a weak acid $AH$ or $BH^+$ and the acidity constant will be positive
The strength of acids or bases can be directly measured only in a narrow range of $pKa (BH^+).$ Outside the range, basicity will be determined by indirect methods. Values of $pka (BH^+)$ outside the interval from $-2$ to $17$ will be inaccurate.
Correlation between structure and strength of acids
The relative strength of acids can be predicted based on the nature of the central atom and the structure of the acid molecule.
The strength of the oxygen-free acid $HX$ and $H_2X$ (where $X$ is a halogen) is higher, the weaker the $X-H$ bond, that is, the larger the radius of the $X$ atom.
In the series $HF - HCl - HBr - HI$ and $H_2S - H_2Se - H_2Te$, the strength of the acids increases.
For oxygen-containing acids, the greater the value of m in the compound of composition $E(OH)nOm$, the higher the strength of the acids.
Thus, according to this theory An acid is any substance whose molecules (including ions) are capable of donating a proton, i.e. be a proton donor; A base is any substance whose molecules (including ions) are capable of attaching a proton, i.e. be a proton acceptor; An ampholyte is any substance that is both a donor and an acceptor of protons.
This theory explains the acid-base properties of not only neutral molecules, but also ions. An acid, giving up a proton, turns into a base, which is the conjugate of this acid. The terms "acid" and "base" are relative concepts, since the same particles - molecules or ions - can exhibit both basic and acidic properties, depending on the partner.
During protolytic equilibrium, acid-base pairs are formed. According to the proton theory, hydrolysis, ionization and neutralization reactions are not considered as a special phenomenon, but are considered to be the usual transfer of protons from an acid to a base.
Particle A formed after the separation of a hydrogen ion
pH value
Water, as a weak electrolyte, undergoes ionization to a small extent:
H 2 O ↔ H + + OH - .
Ions in aqueous solution undergo hydration (aq.)
Water is characterized by protolytic amphotericity. The self-ionization reaction (autoprotolysis) of water, during which a proton from one water molecule (acid) passes to another water molecule (base) is described by the equation:
H 2 O + H 2 O ↔ H 3 O + + OH - .
The equilibrium constant of water autoprotolysis is equal to:
The law of mass action is applied to the ionization constant:
where a is activity.
For brevity, instead of H 3 O + in acid-base equilibrium we write
Since water is in solution in large excess and undergoes ionization to a small extent, it can be noted that its concentration is constant and equal to 55.6 mol (1000 g: 18 g/mol = 56 mol) per liter of water.
Therefore, the product of K and (H 2 O) and the water concentration are equal to 1.8 10 -16 mol/l 55.6 mol/l = 10 -14 mol 2 /l 2. Thus, = 10 -14 (at 25 °C) is a constant value, denoted by Kw and is called water autoprotolysis constant. Sometimes the outdated name is used - the ionic product of water.
Solutions in which the concentration of hydrogen ions and hydroxide ions are the same are called neutral solutions = = = 10 -7 mol/l. In acidic solutions > , > 10 -7 mol/l, and in alkaline solutions > , > 10 -7 mol/l.
To simplify, we take as a basis the hydrogen indicator pH - the decimal logarithm of the concentration of hydrogen ions, taken with the opposite sign: pH = -lg.
Interesting facts:
Violation of the isohydric state ( pH constancy) observed in cardiovascular diseases, ischemia, diabetes mellitus (acidosis develops). Acid-base balance is maintained by breathing, urination, and sweating. These systems work slowly, and immediate neutralization of acidic and alkaline metabolic products is carried out by the body's buffer systems. The state of isohydry is ensured by the combined action of a number of physicochemical and physiological mechanisms. The buffering effect is achieved by combining several protolytic equilibria.
The strength of acids is determined by their ability to donate a proton. The measure of this ability is acidity constant (Ka).
The higher the acidity constant, the stronger the acid. For example, acetic acid is stronger than hydrocyanic acid, since Ka(CH 3 COOH) = 1.74 10 -5, Ka(HCN) = 1 10 -9. For convenience of calculations and recording, they often use not the constants themselves, but their negative decimal logarithms: pKa = -lgKa. The pKa value is called strength indicator of acid. The higher the pKa value, the weaker the acid.
Strong acids almost completely donate their proton to water molecules, so the acid present in the solution is actually a hydronium ion.
In this regard, when calculating the pH of a solution of a strong monobasic acid, the concentration of protons is equated to the concentration of the acid
c(H3O+) = c(HB).
In solutions of weak acids, the concentration of hydronium ions is significantly lower than the concentration of the acid. It is calculated based on
both sides of this equation gives a formula for calculating the pH of solutions of weak acids: pH = 0.5(pKa - log c(HB)).
29. Weak electrolytes. Acidity and basicity constant. Oswald's law of dilution.
Weak electrolytes are chemical compounds whose molecules, even in highly dilute solutions, are slightly dissociated into ions that are in dynamic equilibrium with undissociated molecules. Weak electrolytes include most organic acids and many organic bases in aqueous and non-aqueous solutions.
Weak electrolytes are:
almost all organic acids and water;
some inorganic acids: HF, HClO, HClO 2, HNO 2, HCN, H 2 S, HBrO, H 3 PO 4, H 2 CO 3, H 2 SiO 3, H 2 SO 3, etc.;
some poorly soluble metal hydroxides: Fe(OH) 3, Zn(OH) 2, etc.
Acid dissociation constant (Ka) is the equilibrium constant of the reaction of acid dissociation into a hydrogen ion and an anion of the acid residue. For polybasic acids, the dissociation of which occurs in several stages, separate constants are used for different stages of dissociation, denoting them as K a1, K a2, etc.
Example of dibasic acid calculation:

![]()
More often, instead of the dissociation constant K itself, the value pK is used, which is defined as the negative decimal logarithm of the constant itself:
A base is a chemical compound that can form a covalent bond with a proton (Brønsted base) or with a vacant orbital of another chemical compound (Lewis base). In a narrow sense, bases mean basic hydroxides - complex substances, upon dissociation of which in aqueous solutions, only one type of anion is split off - hydroxide ions OH-.
The Brønsted-Lowry theory allows us to quantify the strength of bases, that is, their ability to abstract a proton from acids. This is usually done using the basicity constant Kb - the equilibrium constant of the reaction of a base with a reference acid, for which water is chosen. The higher the basicity constant, the higher the strength of the base and the greater its ability to abstract a proton. Often the basicity constant is expressed as the basicity constant exponent pKb. For example, for ammonia as a Brønsted base we can write:

Ostwald's dilution law is a relationship expressing the dependence of the equivalent electrical conductivity of a dilute solution of a binary weak electrolyte on the concentration of the solution: ![]()
Here K is the electrolyte dissociation constant, c is the concentration, λ and λ∞ are the values of equivalent electrical conductivity, respectively, at concentration c and at infinite dilution. The relationship is a consequence of the law of mass action and the equality where α is the degree of dissociation.
30. Water is a weak electrolyte. Ionic product of water. PH. POh
Ionic product of water is the product of the concentrations of hydrogen ions H+ and hydroxyl ions OH− in water or in aqueous solutions, the autoprotolysis constant of water.
Water, although a weak electrolyte, dissociates to a small extent:
The equilibrium of this reaction is strongly shifted to the left. The dissociation constant of water can be calculated using the formula:
![]()
Concentration of hydronium ions (protons);
Hydroxide ion concentration;
Concentration of water (in molecular form) in water;
The concentration of water in water, taking into account its low degree of dissociation, is practically constant and amounts to (1000 g/l)/(18 g/mol) = 55.56 mol/l.
At 25 °C, the dissociation constant of water is 1.8·10−16 mol/l. Equation (1) can be rewritten as:
Let us denote the product K· = K in = 1.8·10−16 mol/l·55.56 mol/l = 10−14 mol²/l² = · (at 25 °C).
The constant K in, equal to the product of the concentrations of protons and hydroxide ions, is called the ionic product of water. It is constant not only for pure water, but also for dilute aqueous solutions of substances. With increasing temperature, the dissociation of water increases, therefore, Kv also increases, with decreasing temperature - vice versa.
Hydrogen index, pH - a measure of the activity of hydrogen ions in a solution, and quantitatively expressing its acidity, is calculated as the negative (taken with the opposite sign) decimal logarithm of the activity of hydrogen ions, expressed in moles per liter:
The inverse pH value is somewhat less widespread - an indicator of the basicity of the solution, pOH, equal to the negative decimal logarithm of the concentration of OH - ions in the solution:
Linking level:
In the general case, in accordance with the Bronsted-Lowry protolytic theory, according to equation (4.2) we have for the dissociation of a weak monoprotic acid:
True thermodynamic constant TO this balance will be
where all activities are equilibrium. Let's imagine this ratio in the form:
Let us denote, as in the previous case, the product of two constants TO and a(H 2 O) through (H 2 O) = const at T= const. Then
or approximately:
where all concentrations are equilibrium. Here the value TO A called acid dissociation (ionization) constant or simply acidity constant.
For many weak acids the numerical values TO A are very small, so instead of the size TO A apply strength indicator (or simply indicator):
rK A =- lg TO A .
The more TO A(i.e., the less p TO A ), the stronger the acid.
Let the initial concentration of monobasic acid HB be equal to the degree of its dissociation (ionization) in solution. Then the equilibrium concentrations of the ions [H 3 O + ] and [B - ] will be equal to [H 3 O + ] = [B - ] = αс A , a equilibrium acid concentration [НВ] = With A - α With A = With A(1 - α). Substituting these values of equilibrium concentrations into the expression for the equilibrium constant (4.10), we obtain:
If instead of concentration With A use its inverse V- dilution (dilution), expressed in l/mol, V=1/With A , then the formula for TO A will look like:
This relation and also the expression
describe Ostwald's law of dilution (or dilution law) for a weak binary electrolyte. At a1 (a typical case in many analytical systems)
It is easy to show that, in the general case, for a weak electrolyte of any composition K n A m, which decomposes into ions according to the scheme
K n A m = P TO t+ +t A n -
Ostwald's dilution law is described by the relation
Where With- the initial concentration of a weak electrolyte, for example, a weak acid. So, for orthophosphoric acid H 3 PO 4 (P = 3,
T= 1), which totally decays into ions according to the scheme

![]() .
.
For a binary electrolyte, the relation becomes (4.11). For a1 we have:
Let us find the equilibrium pH value of a solution of monobasic acid NV. Equilibrium concentration of hydrogen ions
Using the notation and we get:
pH = 0.5(r TO A+p With A). (4.12)
Thus, to calculate the equilibrium pH value of a solution of a weak monoprotic acid, it is necessary to know the acidity constant of this acid TO A and its initial concentration With A .
Let's calculate the pH of a solution of acetic acid with an initial concentration of 0.01 mol/l.
At room temperature for acetic acid TO A = 1.74·10 -5 and p TO A = 4,76.
According to formula (4.12) we can write:
pH = 0.5(p TO A+p With A) = 0,5(476-0,01) = 0,5(4,76+2) = 3,38.
A similar consideration can be carried out for equilibria in a solution of any weak polybasic acids.
Polybasic acids dissociate into ions stepwise, in several stages, each of which is characterized by its own equilibrium constant stepwise acid dissociation constant. For example, in solutions of orthoboric acid H 3 BO 3 equilibria are established (the constant values are given for 25 °C):
H 3 VO 3 + H 2 O = H 3 O + +, TO 1
= ![]()
H 2 O = H 3 O + +, TO 2
= ![]()
H 2 O = H 3 O + +, TO 3 =
The acid dissociation constant of each subsequent step is less than the dissociation constant of the previous step - usually by several orders of magnitude.
The product of all stepwise dissociation constants is equal to the total acid dissociation constant K:
TO 1 TO 2 ...TO P =K.
Thus, it is easy to see that for orthoboric acid the value
TO 1
TO 2
TO 3
=K= ![]()
there is a complete acid dissociation constant according to the scheme:
4.3.2 Basicity constant and pH of solutions of weak bases
In accordance with the Brønsted-Lowry protolytic theory of acids and bases, in the general case, for the ionization of a single-acid weak base B in aqueous solutions, we can write:
B + H 2 O = HB + + OH -
If the degree of ionization of the base is a1, then the concentration constant can be taken as the constant of this chemical equilibrium
Proceeding similarly to the previous one, we get:
TO = =K b = const when T= const
as the product of two constants TO=const and [H 2 O] = const.
Let's call the quantity K b , equal, therefore,
K b = , (4.13)
dissociation (ionization) constant of a weak one-acid baseorjust a basicity constant this base, and the magnitude
p K b = K b ,
A strength indicator (or simply an indicator) of the basicity constant.
According to the Ostwald dilution law in the case under consideration (similar to relation (4.11))
K b =,
where is the degree of ionization of a one-acid weak base, and is its initial concentration. Since for a weak base a1, then
Let us find the equilibrium pH value of an aqueous solution of the monoacid base in question at room temperature. In accordance with formula (4.7) we have:
pH = p TO w - pOH = 14 - pOH.
Let's determine the value pOH = [OH - ]. Obviously
[OH - ] = = ![]()
Using the indicators pOH = [OH - ], p TO b =K b And
p = , we get: pOH = 0.5(p TO b+ p). Substituting this expression into the above formula for pH, we arrive at the relation
pH = 14 - pOH = 14 – 0.5 (p TO b+ p).
So, the equilibrium pH value in a solution of a weak one-acid base can be calculated using formula (4.15):
pH = 14 - 0.5(p TO b+ p). (4.15)
Let us calculate the pH in a 0.01 mol/l aqueous solution of ammonia, for which at room temperature TO b= and p TO b = 4,76.
In an aqueous solution of ammonia, an equilibrium is established:
which is mostly shifted to the left, so that the degree of ionization of ammonia is . Therefore, to calculate the pH value, you can use relation (4.15):
pH = 14 - 0.5(p TO b+ p) =
A similar consideration can be carried out for any weak polyacid grounds. True, this results in more cumbersome expressions.
Weak polyacid bases, like weak polybasic acids, dissociate stepwise, and each step of dissociation also has its own stepwise dissociation constant of the base - stepwise basicity constant.
For example, lead hydroxide Pb(OH) 2 in aqueous solutions decomposes into ions in two stages:
![]()
The same equilibria can be written in another way, adhering (within the framework of the protolytic theory) to the definition of a base as a substance that attaches a proton, in this case, accepting it from a water molecule:
The stepwise basicity constants can be represented in the form:
![]()
![]()
With this recording of the indicated equilibria, it is assumed that a proton from a water molecule passes to a hydroxyl group with the formation of a water molecule (), as a result of which the number of water molecules near the lead (II) atom increases by one, and the number of hydroxyl groups associated with the lead (II) atom ), also decreases by one at each dissociation step.
Work TO 1 TO 2 =K=[Pb 2+ ][OH - ] 2 /[Pb(OH) 2 ] =
2.865, where TO- total dissociation constant according to the scheme
or according to a different scheme written down
which ultimately leads to the same result.
Another example is the organic base ethylenediamine, which undergoes ionization in an aqueous solution in two stages. First stage:
![]()
Second stage:
![]()
Work ![]() -
-
total dissociation constant. It corresponds to equilibrium
The numerical values of the equilibrium constants are given above for room temperature.
As in the case of polybasic acids, for a weak polyacid base the dissociation constant of each subsequent step is usually several orders of magnitude less than the dissociation constant of the previous stage.
In table Table 4.2 shows the numerical values of the acidity and basicity constants of some weak acids and bases.
Table 4.2. True thermodynamic ionization constants in aqueous solutions of some acids and bases.
TO A- acidity constant, TO b- basicity constant,
TO 1 - dissociation constant for the first step,
TO 2
- dissociation constant for the second step, etc.
| Dissociation constants of weak acids |
||
| Acid | TO A | R TO A=-lg TO A |
| Nitrogenous Aminoacetic Benzoinaya Boric (orthoboric) Tetraboric Types of protolytic reactions. MU "Solutions" pp. 52-55 Autoprotolysis of water. Ionic product of water.MU "Solutions"» page 56 A small proportion of water molecules are always in an ionic state, although it is a very weak electrolyte. Ionization and further dissociation of water, as already mentioned, is described by the equation of the protolytic reaction of acid-base disproportionation or autoprotolysis. Water is a very weak electrolyte, therefore the conjugate acid and conjugate base formed are strong. Therefore, the equilibrium of this protolytic reaction is shifted to the left. The constant of this equilibrium K equals = The quantitative value of the product of water ion concentration × is ionic product of water. It is equal to: × = K equal. × 2 = 1×10 – 14 Therefore: KH 2O = × = 10 – 14 or simplified KH 2O = × = 10 – 14 KH2O is the ionic product of water, the autoprotolysis constant of water, or simply the constant of water. KH2O depends on temperature. It increases with increasing temperature. In chemically pure water = = = 1×10 – 7. This is a neutral environment. The solution may contain > – the medium is acidic or< – среда щелочная = ; = pH value To quantitatively express the acidity of solutions, use hydrogen ion concentration indicator pH. The hydrogen index is a value equal to the negative decimal logarithm of the concentration of free hydrogen ions in a solution. pH = – log ⇒ = 10 – pH In a neutral environment pH = 7 At acidic pH< 7 In alkaline pH > 7 To characterize the basicity of the medium, the hydroxyl indicator pOH is used рОН = – log [ОH - ] ⇒ [ОH - ] = 10 – рОН pH + pOH = 14 Þ pH = 14 – pOH and pOH = 14 – pH Formulas for calculating pH for solutions of acids and bases. pH = – log
Calculate the pH of a HCl solution with C(HCl) = 0.1 mol/l under the condition of its complete dissociation. C(HCl) = 0.1 mol/l; pH = – log 0.1 = 1 2. Strong bases: [ОH - ] = С(1/z base) Calculate the pH of the NaOH solution under the same conditions. C(NaOH) = 0.1 mol/l; = = 10 – 13 ; pH = – log 10 – 13 = 13 3. Weak acids Calculate the pH of a solution of acetic acid with a molar concentration of 0.5 mol/L. K CH 3COOH = 1.8×10 – 5. 3×10 – 3 pH = – log 3×10 – 3 = 2.5 4. Weak foundations Calculate the pH of an ammonia solution with a molar concentration of 0.2 mol/L. K NН 3 = 1.76×10 – 5 1.88×10 – 3 0.53×10 – 11; pH = – log 0.53×10 – 11 = 11.3 5. C(H +) = [H + ] = 10 – pH At pH = 7, [H + ] = 10 – 7 There are various methods for determining pH: using indicators and ionomer devices. The value of pH for chemical reactions and biochemical processes in the body. Many reactions require a strictly defined pH value to proceed in a certain direction. Normally, in a healthy body, the reaction of the environment of most biological fluids is close to neutral. Blood – 7.4 Saliva – 6.6 Intestinal juice – 6.4 Bile – 6.9 Urine – 5.6 Gastric juice: a) at rest – 7.3 b) in a state of digestion – 1.5-2 Deviation of pH from the norm has diagnostic (definition of the disease) and prognostic (course of the disease) significance. Acidosis is a shift in pH to the acidic side, the pH decreases, the concentration of hydrogen ions increases. Alkalosis is a shift in pH to the alkaline region, the pH increases, and the concentration of hydrogen ions decreases. A temporary deviation of blood pH from the norm by tenths leads to serious disturbances in the body. Long-term deviations in blood pH can be fatal. Deviations in blood pH can be 6.8 - 8; changes outside this range in any direction are incompatible with life. Combined and isolated protolytic equilibria. Protolytic processes are reversible reactions. Protolytic equilibria are shifted towards the formation of weaker acids and bases. They can be considered as competition between bases of different strengths for the possession of a proton. They talk about isolated and combined equilibria. If several simultaneously existing equilibria are independent of each other, they are called isolated. A shift in equilibrium in one of them does not entail a change in the equilibrium position in the other. If a change in equilibrium in one of them leads to a change in equilibrium in the other, then we speak of combined (conjugate, competing) equilibria. The predominant process in systems with combined equilibrium is the one characterized by a larger value of the equilibrium constant. The second process will be predominant, because its equilibrium constant is greater than the equilibrium constant of the first process. The equilibrium in the second process is shifted to the right to a greater extent, because methylamine is a stronger base than ammonia, NH 4 + is a stronger acid than CH 3 NH 3 +. Conclusion: A stronger base suppresses the ionization of a weaker base. Therefore, when a small amount of hydrochloric acid is added to a mixture of ammonia and methylamine, it will be mainly the methylamine that undergoes protonation. And also: the strongest acid suppresses the ionization of weak acids. Thus, hydrochloric acid found in gastric juice suppresses the ionization of acetic acid (coming from food) or acetylsalicylic acid (medicinal substance). ______________________________________________________________ Related publications
| ||