Lyrica - official * instructions for use. Lyrics - official* instructions for use Writes out lyrics
Many drugs manage to misuse, including Lyrica, which, acting like a drug, has its own consequences of use, side effects and has its effect on the mind and body of the addict.
What is Lyrica?
Lyrica tablets is the trade name for a drug primarily used to treat epilepsy. The tool was first released at the beginning of the century in the United States, since then they have been actively treated all over the world for conditions such as:
- Anxiety.
- Fibromyalgia and simple myalgia.
- Chronic muscle and "bone" pain.
- epilepsy.
The active chemical in the drug is pregabalin. The same substance and, accordingly, a similar effect, have drugs produced by German pharmacologists - Algerica, Pregabalin-Richter.
Narcotic effect
Having tasted Lyrica, drug addicts "unanimously" issued a verdict - this medicine perfectly relieves withdrawal symptoms. This is true, the active substance of the drug has such an effect in the withdrawal state:
- removes anxiety;
- relieves physical pain;
- relieves spasms and "twisting".
However, when used under the supervision of a narcologist, the drug is strictly dosed, and is accompanied by additional therapy, which the addict is not able to do on his own, without the help of a specialist. Usually, the addicted person does not read the instructions at all, just releases the pills from the package and swallows them.
As a result, the withdrawal really goes away, but Lyrica itself begins to act like a drug:
- Brings complete peace.
- Gives a feeling of absolute well-being.
- It pushes back, removes all problems and negativity from consciousness.
And so on. The side effects of the drug are similar to those of heroin, and dependence, already on Lyrica, occurs very quickly. The drug addict seems to stop taking drugs, but in fact, he simply changes one for another, although outwardly he is only taking the medicine.
Addiction
Medicines that are used in psychology, neurology and similar fields can form a strong addiction even in a person who is completely healthy, who does not smoke or drink. This drug is no exception, the "lyrical" effect is so attractive that the human brain wants to experience it again and getting high becomes not just an obsession, it is generally the only purpose for which the patient wakes up.
Even an experienced doctor cannot always determine the moment when the drug recommended by him began to act not as a medicine, but as a drug. Sick people are extremely inventive and go to great lengths to increase the dosage of the remedy and justify it if it is under control. For example, they imitate convulsive seizures, muscle pain, and so on.
Speaking of drug addicts who first used the drug as a panacea for withdrawal, a new addiction for them is formed instantly, since the use of Lyrica has full psychoactive and chemical effects. At the same time, relatives and relatives are always supportive of a new hobby, sincerely believing that a person has "jumped off", and financially the drug is much cheaper.
However, with a dense and long application, everything becomes obvious. The following symptoms appear, indicating the presence of dependence on the drug:
- Coordination in possession of one's own body disappears - spontaneous staggering occurs when walking, meaningless jerky movements when a person is standing or sitting.
- Spontaneously begin to "run" eyes, without objective reasons.
- The pupil is constantly dilated, regardless of the lighting.
- Increased and very profuse perspiration with a strong odor.
- Confused consciousness, a person is lost in calendar dates, time of day, etc.
- There are flashes of uncontrollable aggression both against people or animals, and against inanimate objects - a drug addict in a state of attack is able to break a sofa, for example.
- Characterized by mood swings and sleep disturbances.
Quitting Lyrica isn't easy at all, it's not "gastric lavage" and "no pills to swallow" anymore. Breaking from the absence of the drug is similar to heroin, and it is simply not realistic to survive it on your own. With the formed dependence on the drug or its overdose in the body, help from specialists and full-fledged treatment with rehabilitation are needed.
Treatment
Quitting drug abuse is nothing new and will require a standard long course of drug treatment. The success of getting rid of this addiction, just like any other, consists of the main key points:
- Awareness by the patient of his own state and his desire to change this state.
- Just like with heroin addiction, outpatient procedures have no effect, it is necessary to place the person in a hospital.
- Detoxification of the body under a dropper and stimulation of metabolism with the help of saline solutions and drugs.
- Injections of painkillers and sedatives.
- The introduction of minerals and, of course, vitamins, in a medical form, for instant absorption in order to maintain the brain and the whole organism as a whole.
- Treatment by a psychologist in order to remove the psychological aspect of addiction.
- A complete comprehensive examination of a person, to identify any complications or pathologies.
Inpatient treatment lasts individually, but in less than six months, the consequences of using Lyrica cannot be overcome.
Video: Lyrics - bringing death.
Side effects and contraindications
Since ordinary people who are not at all striving to get high often become drug addicts due to taking drugs, before taking a drug containing pregabalin recommended by a doctor, you need to carefully study all its side effects and get acquainted with contraindications.
Signs of side effects include:
- Difficulties with digestion - belching, flatulence in combination with constipation, dry mouth.
- Breathing problems - sudden cough, shortness of breath for no reason, dry nose, choking and shortness of breath.
- Change the perception of reality from depressive to euphoric in minutes.
- Tremor, both in the hands and feet.
- Feeling that muscles, calves, organs are trembling or shaking.
- Impaired vision - the image on the screen or monitor doubles, objects “leave” such as houses or trees on the street.
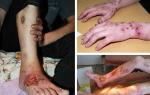
- A focal skin rash appears in the form of large round red abscesses.
- There are attacks of sweating with a pungent odor and a change in foci of excretion - from the palms or feet, to the whole body.

If at the beginning of treatment with Lyrica such side effects, at least one of them, appear, you should immediately stop taking the medicine and contact your doctor. It is impossible to reassure yourself that this is a side effect, and it will pass. Since this will not pass, but will develop into the formation of a persistent and severe addiction that will cripple both life and health.
As for contraindications, they are as follows:
- Difficulties in metabolism, poor absorption of galactose and glucose by the body.
- Pronounced mental disorders of personality.
- All pathologies associated with the central nervous system.
- Pregnancy and lactation.
- Malfunctions in the cardiovascular system.
- Kidney disease, liver disease.
- Unformed, growing organisms - that is, up to 17-19 years old, the medicine is not prescribed.
- Intolerance to the active substance of the drug.
The difficulty is that contraindications are not always obvious. For example, a person of 25-30 years old simply may not know about the presence of hidden pathologies in the heart, kidneys or liver, and the doctor is not a magician, and is guided by the data indicated in the medical record and the words of the patient himself.
The same applies to allergic intolerance, it is not determined before the start of the medication. In the event of any side effects, allergic phenomena, or pain in the heart and other organs, Lyrica should be stopped and the doctor should be informed.
Sometimes, dependence on the drug can occur from the very first dose, in this case, when treatment is stopped, the following appear:
- most signs of colds - from fever to runny nose;
- nausea;
- uncontrollable diarrhea;
- depressive mood;
- compressive migraines.
The attending doctor needs to know about the occurrence of these symptoms, they cannot be neglected.
In general, Lyrica, despite its poetic name, is a serious medicine, the use of which can aggravate the patient's condition and lead to new problems. Therefore, it is impossible to arbitrarily and uncontrollably use this drug, since at any time it can become a drug from a medicine.
| Lyrica
Analogues (generics, synonyms)
Pregabalin-Richter


Recipe (International)
Temporarily unavailable
pharmachologic effect
Lyrica is a drug with antiepileptic and anticonvulsant activity. The active component of the drug is pregabalin, an alkylated analogue of gamma-aminobutyric acid, however, despite the structural similarity of the molecules, pregabalin does not have the activity characteristic of gamma-aminobutyric acid. Pregabalin has neither direct nor mediated GABAergic effects. The mechanism of action of the drug is based on its ability to bind to alpha-2-delta subunits of neuronal calcium channels (N- and P / O-type calcium channels), as a result of which there is a decrease in calcium transport into neuronal cells in response to an action potential. The drug is characterized by a high degree of affinity for alpha-2-delta protein, located in the tissues of the central nervous system. The use of the drug leads to a decrease in the release of pain neurotransmitters (including glutamate, norepinephrine and substance P) into the synaptic cleft during excitation of neurons. Due to such changes, under the influence of the drug, impulse conduction is selectively suppressed; it should be noted that Lyrica suppresses the excitability of the neuron network only in pathological conditions.
The drug has an analgesic effect for pain of neuropathic etiology and postoperative pain syndrome, including conditions such as hyperalgesia and alodynia.
The drug at a therapeutic dose is well tolerated by patients; in the course of studies, the absence of a teratogenic effect was noted when using the drug at doses 2 times higher than the therapeutic dose. As a result of a number of studies, the absence of carcinogenic and genotoxic effects of pregabalin was noted.
After oral administration, the drug is well absorbed in the gastrointestinal tract, the peak concentration of the active substance in the blood plasma is observed 1 hour after oral administration of the drug. With repeated use of Lyrica, the time to reach the maximum plasma concentration of the active substance does not change. The bioavailability of the drug does not depend on the dose taken and is about 90%. With repeated use of the drug, equilibrium concentrations of pregabalin are achieved within 24-48 hours. Food intake reduces the rate and degree of absorption of pregabalin, so while taking the drug with food, the time to reach the peak of the active substance in plasma increases by 2.5 hours, and the maximum plasma concentrations of the drug decrease by 25-30% (compared to the data obtained after taking drug on an empty stomach). It should be noted that food intake does not have a clinically significant effect on the amount of absorption.
The drug is not characterized by binding to plasma proteins. Pregabalin penetrates well through the blood-brain and hematoplacental barriers, and is also excreted in breast milk.
An insignificant part of the drug (less than 1%) is metabolized to form an N-methylated compound. The drug is excreted unchanged in the urine. About 1% of the drug is excreted in the urine as a metabolite. The half-life of the drug is 6.3 hours. It should be noted that pregabalin clearance is directly proportional to creatinine clearance.
In patients taking antiepileptic drugs and patients suffering from chronic pain, pharmacokinetics are similar to those in healthy volunteers.
In patients with impaired renal function, dose adjustment is necessary. The decrease in pregabalin clearance in patients with renal insufficiency is directly proportional to the decrease in creatinine clearance. Patients with reduced creatinine clearance need to reduce the dose of Lyrica, and patients on hemodialysis, after a hemodialysis session, it is necessary to increase the dose of pregabalin (after 4 hours of hemodialysis, about 50% of the accepted dose of the drug is excreted from the blood plasma).
Patients suffering from hepatic insufficiency, dose adjustment of the drug is usually not required, since pregabalin is metabolized in small quantities, and impaired liver function does not significantly affect the pharmacokinetics of the drug.
In elderly patients, there is a decrease in creatinine and pregabalin clearance, it is recommended to adjust the dose of Lyrica in patients with age-related impaired renal function.
Mode of application
For adults: Inside, regardless of food intake, the daily dose of Lyrica is 150-600 mg, divided into 2-3 doses.
Neuropathic pain: initial dose - 150 mg / day; depending on the effect achieved and tolerability, after 3-7 days, the dose of Lyrica is increased to 300 mg / day, if necessary, after another 7 days - up to a maximum dose of 600 mg / day.
With epilepsy: initial dose - 150 mg / day; depending on the effect achieved and tolerability, after 7 days the dose is increased to 300 mg / day, after 7 days - up to a maximum dose of 600 mg / day.
In case of renal insufficiency, the daily dose of Lyrica is selected taking into account CC: with CC 60 ml / min and more, the initial dose is 150 mg / day, the maximum is 600 mg / day, the frequency of administration is 2-3 times; with CC 30-60 ml / min, the initial dose is 75 mg / day, the maximum is 300 mg / day, the frequency of administration is 2-3 times; with CC 15-30 ml / min, the initial dose is 25-50 mg / day, the maximum is 150 mg / day, the frequency of administration is 1-2 times; with CC less than 15 ml / min, the initial dose is 25 mg / day, the maximum dose of Lyrica is 75 mg / day, the frequency of administration is 1 time.
With hemodialysis, after each 4-hour session, an additional initial dose is prescribed - 25 mg / day, maximum - 100 mg / day once.
Patients over 65 years of age may require a dose reduction due to decreased renal function.
Treatment with Lyrica is discontinued gradually over a period of at least 1 week.
Indications
The drug is used to relieve pain in patients suffering from fibromyalgia and pain of neuropathic etiology.
In addition, the drug is used to treat patients with generalized anxiety disorders and epilepsy.
In patients with epilepsy, Lyrica is used as an adjunctive therapy for partial (partial) seizures, including partial seizures that are accompanied by secondary generalization.
Contraindications
Hypersensitivity to the components of Lyrica, children's age (up to 17 years), lactation period; for LF containing lactose - galactose intolerance, Lapa lactase deficiency, malabsorption of glucose / galactose.
Side effects
The drug is usually well tolerated by patients, however, in isolated cases, the development of the same side effects is possible (it is possible that the development of side effects is associated with the course of the underlying disease):
On the part of the gastrointestinal tract: appetite disorders (both increased appetite and anorexia are possible), dry mouth, nausea, vomiting, stool disorders, flatulence. In addition, hypersalivation, oral hypoesthesia, and gastroesophageal reflux may develop. In isolated cases, patients developed pancreatitis, hypoglycemia, ascites, and dysphagia.
From the side of the cardiovascular system and the hematopoietic system: tachycardia, neutropenia, flushing and flushing, atrioventricular blockade of the first degree, changes in blood pressure. In isolated cases, the development of sinus arrhythmia is possible (the development of both tachycardia and bradycardia was noted).
From the side of the central and peripheral nervous system: dizziness, headache, drowsiness, ataxia, decreased attention, impaired coordination, euphoria, irritability, confusion. In addition, it is possible to develop a violation of mnestic function, memory loss, tremor, dysarthria, paresthesia, speech disorders, difficulty in choosing words, anxiety, depersonalization. Some patients have developed sleep disorders, including insomnia and unusual dreams. When using the drug, patients experienced sudden mood swings, depression, unreasonable anxiety, hallucinations, apathy, high spirits, psychomotor hyperactivity, hyperesthesia, decreased reflexes, dyskinesia, and the development of an acute anxiety state with a panic reaction. In isolated cases, the development of hypokinesia, diplopia and parsomia is possible.
From the senses: impaired taste sensitivity, hyperacusis, visual disturbances, including diplopia, dry eyes, decreased visual acuity, lacrimation, asthenopia, eye pain. In isolated cases, the development of photopsia, eye irritation, mydriasis, impaired peripheral vision, oscillopsia and strabismus was noted.
From the respiratory system: respiratory failure, dry mucous membranes, cough, rhinitis, nasopharyngitis, snoring. In addition, nosebleeds and a feeling of constriction in the throat may develop.
From the musculoskeletal system: muscle fasciculations, convulsions, pain in muscles and joints, swelling of the joints, muscle rigidity, pain in the back and limbs. In isolated cases, the development of spasm of the neck muscles and rhabdomyolysis was noted.
From the genitourinary system: a decrease in the amount of urine, the development of renal failure, urinary incontinence, erectile dysfunction, ejaculation disorders, amenorrhea, dysmenorrhea, a decrease or increase in libido, anorgasmia.
Allergic reactions: skin rash, itching, urticaria, papular rash.
Others: increased sweating, soreness and hypertrophy of the mammary glands, discharge from the mammary glands.
When using the drug, there is a change in some indicators of laboratory tests, including an increase in the activity of hepatic transferases, an increase in the concentration of creatinine and glucose in the blood, a decrease in the number of platelets and leukocytes in the blood, hypokalemia.
Release form
ATTENTION!
The information on the page you are viewing was created for informational purposes only and does not promote self-treatment in any way. The resource is intended to familiarize healthcare professionals with additional information about certain medicines, thereby increasing their level of professionalism. The use of the drug "" without fail provides for a consultation with a specialist, as well as his recommendations on the method of application and dosage of the medicine you have chosen.
The Ministry of Health signed and submitted to the Ministry of Justice Order No. 634n dated September 10, 2015 “On making some changes to some orders of the Ministry of Health and Social Development of the Russian Federation and the Ministry of Health of the Russian Federation”. Letters with this information were sent to the heads of regional health authorities. The order supplements the list of medicines for medical use subject to subject-quantitative accounting.
The specified order regulates the inclusion in the list of medicines for medical use subject to subject-quantitative accounting, approved by order of the Ministry of Health of Russia dated April 22, 2014 No. 183n, of new positions of drugs under the international generic names pregabalin, tropicamide and cyclopentolate.
Only now the Ministry of Health has been able to implement an idea that has been discussed for several years - it has tightened the conditions for the sale of pregabalin (antiepileptic tablets "Lyric", "Algerica", "Pregabalin-Richter" and others), tropicamide (eye drops "Tropicamide") and cyclopentolate (eye drops "Cyclomed").
Lyrica is an antiepileptic drug that is also prescribed for those suffering from neuropathic pain. This medicine is very popular among drug users. Some use it to relieve withdrawal, others - to achieve the effect of drug intoxication. "Tropikamid" and "Cyclomed" - eye drops for medical pupil dilation also fell into the category of "pharmaceutical drugs".
Thus, from October 1, 2015, prescriptions for these drugs must be written in accordance with the requirements of the Order of the Ministry of Health of Russia dated December 20, 2012 No. specified forms, their accounting and storage.
In addition, these drugs will be subject to subject-quantitative accounting by drug manufacturers, drug wholesalers, pharmacies and medical organizations that circulate drugs, individual entrepreneurs in accordance with the requirements of the Order of the Ministry of Health of Russia No. 378n dated June 17, 2013.
Previously, pharmacies had the opportunity to illegally sell drugs without a prescription - everything depended on the conscientiousness of the pharmacist. Now these drugs will be sold under prescriptions of a different form - , and pharmacies will report for each drug sold in accordance with prescriptions. The drugs will have to be stored in wooden or metal lockable cabinets in accordance with the requirements of the Rules for the Storage of Medicines (approved by Order of the Ministry of Health and Social Development of Russia dated August 23, 2010 No. 706n).